 Despite this preparatory work for Minuteman, the missile did not begin formal development until the air force secured final DoD approval in February 1958. Meanwhile, civilian engineers employed by the air force at the Non-Rotating Engine Branch at Wright-Patterson AFB had continued efforts to develop large, solid-propellant motors. Perhaps from sources at Aerojet, they learned about adding large quantities of aluminum to a solid propellant to increase its performance. They combined this with other information they had been gathering on solid propellants while Hall was still with the branch. Bill Fagan from the branch carried this information to Hall at the Ballistic Missile Division (as the WDD had become).52
Despite this preparatory work for Minuteman, the missile did not begin formal development until the air force secured final DoD approval in February 1958. Meanwhile, civilian engineers employed by the air force at the Non-Rotating Engine Branch at Wright-Patterson AFB had continued efforts to develop large, solid-propellant motors. Perhaps from sources at Aerojet, they learned about adding large quantities of aluminum to a solid propellant to increase its performance. They combined this with other information they had been gathering on solid propellants while Hall was still with the branch. Bill Fagan from the branch carried this information to Hall at the Ballistic Missile Division (as the WDD had become).52
The specifics of Minuteman technology continued to evolve, but its basic concept took advantage of the reduced complexity of solids over liquids. This cut the number of people needed to launch it. Each missile could be remotely launched from a control center using communications cables, without a crew of missileers in attendance. The air force initially considered using mobile Minutemen but ultimately decided to launch them from silos. Because solids were more compact than liquids, Minuteman IA (by one account) was only 53.8 feet long to Atlas’s 82.5, Titan I’s 98, and Titan II’s 108 feet in length. Its diameter was slightly over half that of the other three missiles (5.5 feet to the others’ 10), and its weight was only 65,000 pounds to Atlas’s 267,136, Titan I’s 220,000, and Titan II’s 330,000 pounds. All of this made the costs of silos much lower and substantially reduced the thrust needed to launch the missile. Consequently, the initial costs of Minuteman were a fifth and the annual maintenance costs a tenth those of Titan I. Moreover, a crew of two could launch 10 Minutemen, whereas it took six people to launch each Titan I—a 30-fold advantage in favor of Minuteman.53
Minuteman development used multiple approaches to arrive at individual technologies, as had been true with Atlas. Ballistic Missile Division (BMD) contracted separately with Aerojet and Thiokol to work on all three stages of the missile. A later contract with Hercules assigned it to work on the third stage, too. As firms developed technologies, parallel development gave way to specific responsi-
bilities, with Thiokol building the first stage, Aerojet the second, and Hercules the third. Space Technology Laboratories retained its role in systems engineering and technical direction at BMD. These and other companies and organizations all sent representatives to frequent program-review meetings and quarterly gatherings of top officials. They examined progress and identified problems requiring solution. Then, the relevant organizations found solutions to keep the program on track.54
One major technical challenge involved materials for the nozzle throats and exit cones. The addition of aluminum to the propellant provided a high enough specific impulse to make Minuteman feasible, as it had done for Polaris, and its combustion produced aluminum oxide particles that damped instabilities in the combustion 248 chamber. But the hot product flow degraded the nozzle throats and Chapter 6 other exposed structures. It seemed that it might not be possible to design a vectorable nozzle that would last the 60 seconds needed for the missile to reach its ballistic trajectory so it could arrive accurately on target. Solving this problem required “many months and many dollars. . . spent in a frustrating cycle of design, test, failure, redesign, retest, and failure." The Minuteman team used many different grades and exotic compounds of graphite, which seemed the most capable material, but all of them experienced blowouts or performance-degrading erosion. One solution was a tungsten throat insert, a compromise in view of its high weight and cost. For the exit cones, the team tried Fiberite molded at high pressure and loaded with silica and graphite cloth. It provided better resistance to erosion than graphite but still experienced random failures.55
Another significant problem concerned the vectorable feature of the nozzles. Polaris had solved its steering problem with jetavators, but flight-control studies for Minuteman showed a need for the stage-one nozzles to vector the thrust eight degrees, more than Min – uteman engineers thought jetavators could deliver. Thiokol’s test of the motor in 1959—the largest solid-propellant powerplant yet built—resulted in the ejection of all four nozzles after 30 milliseconds of firing, well before the full stage had ignited. Five successive explosions of the motors and their test stands occurred in October 1959, each having a different failure mode. BMD halted first-stage testing in January 1960. Discussion among BMD, STL, and Thiokol personnel revealed two problem areas, internal insulation and the nozzles themselves, as masking other potential problems.
There followed two concurrent programs of testing. Firings with battleship-steel cases tested movable nozzles, while participants used flight-weight cases to test a single, fixed nozzle massive
enough to sustain a full-duration firing. Thiokol solved the problem with insulation by summer but did not resolve the nozzle issue until fall. This was close to the date set for all-up testing of the entire missile. However, General Phillips had ordered Thiokol to begin manufacturing the first stage except the nozzles, permitting installation of the nozzles as soon as their problem was solved.56
 Among many other difficulties, a major concern was launching from a silo. The first successful launch of the missile occurred in early February 1961, which Phillips referred to as “December 63rd" since the planned date had been in December 1960. It and two succeeding launches took place from a surface pad and were so successful that the team advanced the first silo launch to August 1961. The missile blew up in the silo, giving credence to critics in STL who had argued that firing a missile from a silo was impossible. As Phillips said, the missile “really came out of there like a Roman candle."57
Among many other difficulties, a major concern was launching from a silo. The first successful launch of the missile occurred in early February 1961, which Phillips referred to as “December 63rd" since the planned date had been in December 1960. It and two succeeding launches took place from a surface pad and were so successful that the team advanced the first silo launch to August 1961. The missile blew up in the silo, giving credence to critics in STL who had argued that firing a missile from a silo was impossible. As Phillips said, the missile “really came out of there like a Roman candle."57
Fortunately, team members recovered enough of the guidance system from the wreckage to find that the problem was not the silo launch itself but quality control. Solder tabs containing connections had vibrated together, causing all of the stages to ignite simultaneously. Knowing this, the team was able to prepare the fifth missile for flight with the problem solved by mid-November, when it had a successful flight from the silo. Previous testing of silo launches aided this quick recovery. In early 1958, BMD and STL engineers had arranged for development of underground silos. They divided the effort into three phases, with the first two using only subscale models of Minuteman. The third tested full-scale models. Most testing occurred at the air force’s rocket site on Edwards AFB in the remote Mojave Desert, but Boeing (contractor for missile assembly and test) tested subscale models in Seattle.
At the rocket site on Leuhman Ridge at Edwards, subscale silos investigated heat transfer and turbulence in some 56 tests by November 1958. Meanwhile, Boeing modeled the pressures that the rocket’s exhaust gases imparted to the missile and silo. It also examined acoustic effects of the noise levels generated by the rocket motor in the silo on delicate systems such as guidance. Armed with such data, engineers at Edwards began one-third-scale tests in February 1959. Full-scale tests initially used a mock-up made of steel plate with ballast to match the weight and shape of the actual missile and only enough propellant (in the first-stage motor) to provide about three seconds of full thrust—enough to move the missile, on a tether, out of the silo and to check the effects of the thrust on the silo and missile. They configured the tether so that the missile
would not drop back on the silo and damage it. These tests ensured that the second silo launch at Cape Canaveral on November 17, 1961, was fully successful.58
The all-up testing on Minuteman was itself a significant innovation later used on other programs, including the Saturn launch vehicle for the Apollo program. It differed from the usual practice for liquid-propellant missiles—gradually testing a missile’s different capabilities over a series of ranges and tests (for first-stage or booster propulsion, for second-stage or sustainer-engine propulsion, for guidance/control, and so forth). For the first time, it tested all of the missile’s functions at once over the full operational range. Although the practice accorded well with general procedures at BMD, such as concurrency, its use came about in an odd way. According 250 to Otto Glasser, he was briefing Secretary of the Air Force James H.
Chapter 6 Douglas on Minuteman, with Gen. Curtis LeMay, vice chief of staff of the air force, sitting next to Douglas. Douglas insisted Glasser had moved the first flight of the missile to a year later than the original schedule. Glasser protested (to no avail) that this was not the case, and the only way he could conceive to cut a year out of the development process was all-up testing. “Boy, the Ramo-Wooldridge crowd came right out of the chair on that," Glasser said. They protested “a test program. . . with that sort of lack of attention to all normal, sensible standards." But all-up testing “worked all the way."59
Overcoming these and other problems, BMD delivered the first Minuteman I to the Strategic Air Command in October 1962, almost exactly four years after the first contracts had been signed with contractors to begin the missile’s development. This was a year earlier than initially planned because of the speeded-up schedule. The missile in question was the A model of Minuteman I, later to be succeeded by a B model. The former was 53.7 feet long and consisted of three stages plus the reentry vehicle. Thiokol’s first stage included a new propellant binder developed by the company’s chemists from 1952 to 1954. Thiokol first tried a binder called polybutadiene-acrylic acid, or PBAA, an elastomeric (rubberlike) copolymer of butadiene and acrylic acid that allowed higher concentrations of solid ingredients and greater fuel content than previous propellants. It had a higher hydrogen content than earlier Thiokol polysulfide polymers. With PBAA, a favorable reaction of oxygen with the aluminum generated significant amounts of hydrogen in the exhaust gases, reducing the average molecular weights of the combustion products (since hydrogen is the lightest of elements). This added to the performance, with Minuteman being the first rocket to use the new binder.60
But testing showed PBAA had a lower tear strength than polysulfide, so Thiokol added 10 percent acrylonitrile, creating polybutadiene-acrylic acid-acrylonitrile (PBAN). The binder and curing agent constituted only 14 percent of the propellant, with ammonium perchlorate (oxidizer) and aluminum (fuel) the two other major ingredients. The combination yielded a theoretical specific impulse of more than 260 lb-sec/lb, with the actual specific impulse at sea level at 70°F somewhat lower than 230.61
 For stage two of Minuteman I, Aerojet used the polyurethane binder employed in Polaris, with ammonium perchlorate as the oxidizer and aluminum powder the major fuel. It used two slightly different propellant grains, with a faster-burning inner grain and a slower-burning outer one. The combination resulted in a conversion of the four-point, star-shaped, internal-burning cavity to a cylindrical one as the propellant burned, avoiding slivers of propellant that did not burn. The propellant yielded a vacuum specific impulse of nearly 275 lbf-sec/lbm at temperatures ranging from 60°F to 80°F.62
For stage two of Minuteman I, Aerojet used the polyurethane binder employed in Polaris, with ammonium perchlorate as the oxidizer and aluminum powder the major fuel. It used two slightly different propellant grains, with a faster-burning inner grain and a slower-burning outer one. The combination resulted in a conversion of the four-point, star-shaped, internal-burning cavity to a cylindrical one as the propellant burned, avoiding slivers of propellant that did not burn. The propellant yielded a vacuum specific impulse of nearly 275 lbf-sec/lbm at temperatures ranging from 60°F to 80°F.62
For stage three, the Hercules Powder Company used a glass- filament-wound case instead of the steel employed on stages one and two, plus a very different propellant than for the first two stages. The third stage featured four phenolic-coated aluminum tubes for thrust termination and a grain consisting of two separate compositions. The one used for the largest percentage of the grain included the high-explosive HMX, combined with ammonium perchlorate, nitroglycerin, nitrocellulose, aluminum, a plasticizer, and a stabilizer. The second composition had the same basic ingredients minus the HMX and formed a horizontal segment at the front of the motor. A hollow core ran from the back of the motor almost to the segment containing the non-HMX composition. It was roughly cone shaped before tapering off to a cylinder. This motor yielded a specific impulse of more than 275 lbf-sec/lbm at temperatures ranging from 60°F to 80°F. The four nozzles for stage three of Min – uteman I rotated in pairs up to four degrees in one plane to provide pitch, yaw, and roll control.63 Minuteman I, Wing I became operational at Malmstrom AFB, Montana, in October 1962.64
It would be tedious to follow the evolution of Minuteman through all the improvements in its later versions, but some discussion of the major changes is appropriate. Wings II through V of Minuteman I (each located at a different base) featured several changes to increase the missile’s range. This had been shorter than initially planned because of the acceleration of the Minuteman I schedule. The shorter range was not a problem at Malmstrom because it was so far north (hence closer to the Soviet Union), but range became a problem
starting with Wing II. Consequently, for it and subsequent missiles, more propellant was added to the aft dome of stage one, and the exit cone included contouring that made the nozzle more efficient. In stage two, the material for the motor case was changed from steel to titanium. Titanium is considerably lighter than steel but more expensive. Since each pound of reduced weight yielded an extra mile of range, use of titanium seemed worth the extra cost. The nozzles also were lighter. Overall, the reduction in weight totaled slightly less than 300 pounds despite an increase in propellant weight. The increase in propellant mass plus the decrease in weight yielded a range increase of 315 miles to a figure usually given as 6,300 nautical miles. There were no significant changes to stage three.65
 MINUTEMAN II
MINUTEMAN II
For Minuteman II, the major improvements occurred in Aerojet’s stage two. There had been problems with cracking and ejection of graphite from the nozzles and aft closure of stage one. An air force reliability improvement program solved these difficulties. There had also been problems with insulation burning through in the aft dome area of stage three. Unspecified design changes inhibited the flow of hot gases in that region. Stage two, however, featured an entirely new rocket motor with a new propellant, a slightly greater length, a substantially larger diameter, and a single fixed nozzle that used a liquid-injection thrust-vector-control system for directional control.66
The new propellant was carboxy-terminated polybutadiene (CTPB), which propellant companies other than Aerojet had developed. Some accounts attribute its development to Thiokol, which first made the propellant in the late 1950s and converted it into a useful propellant in the early 1960s. Initially, Thiokol chemists used an imine known as MAPO and an epoxide in curing the CTPB. It turned out that the phosphorous-nitrogen bond in the imine was susceptible to hydrolysis, causing degradation and softening of the propellant. According to Thiokol historian E. S. Sutton, “The postcuring problem was finally solved by the discovery that a small amount of chromium octoate (0.02%) could be used to catalyze the epoxide-carboxyl reaction and eliminate this change in properties with time." A history of Atlantic Research Corporation agrees that Thiokol produced the CTPB but attributes the solution of the curing problem to ARC, which is not incompatible with Sutton’s account. According to the ARC history, “ARC used a complex chromium compound, which would accelerate the polymer/epoxy reaction,
paving the way for an all epoxy cure system for CTPB polymer." The result was “an extremely stable binder system."67
 It frequently happens in the history of technology that innovations occur to different people at about the same time. This appears to have been the case with CTPB, which Aerojet historians attribute to Phillips Petroleum and Rocketdyne without providing details. These two companies may have been the source for information about the CTPB that Aerojet used in Minuteman II, stage two. Like Thiokol, in any event, Aerojet proposed to use MAPO as a cross-linking agent. TRW historians state that their firm’s laboratory investigations revealed the hydrolysis problem. They state that “working with Aerojet’s research and development staff," they developed “a formulation that eliminated MAPO. . . ." The CTPB that resulted from what apparently was a multicompany development effort had better fuel values than previous propellants, good mechanical properties such as the long shelf life required for silo – based missiles, and a higher solids content than previous binders. The propellant consisted primarily of CTPB, ammonium perchlorate, and aluminum. It yielded a vacuum specific impulse more than 15 lbf-sec/lbm higher than the propellant used in stage two of Minuteman I, Wing II.68
It frequently happens in the history of technology that innovations occur to different people at about the same time. This appears to have been the case with CTPB, which Aerojet historians attribute to Phillips Petroleum and Rocketdyne without providing details. These two companies may have been the source for information about the CTPB that Aerojet used in Minuteman II, stage two. Like Thiokol, in any event, Aerojet proposed to use MAPO as a cross-linking agent. TRW historians state that their firm’s laboratory investigations revealed the hydrolysis problem. They state that “working with Aerojet’s research and development staff," they developed “a formulation that eliminated MAPO. . . ." The CTPB that resulted from what apparently was a multicompany development effort had better fuel values than previous propellants, good mechanical properties such as the long shelf life required for silo – based missiles, and a higher solids content than previous binders. The propellant consisted primarily of CTPB, ammonium perchlorate, and aluminum. It yielded a vacuum specific impulse more than 15 lbf-sec/lbm higher than the propellant used in stage two of Minuteman I, Wing II.68
Although CTPB marked a significant step forward in binder technology, it was not as widely used as it might have been because of its higher cost compared with PBAN. Another factor was the emergence in the late 1960s of an even better polymer with lower viscosity and lower cost, hydroxy-terminated polybutadiene (HTPB). It became the industry standard for newer tactical rockets. HTPB had many uses as an adhesive, sealant, and coating, but to employ it in a propellant required, among other things, the development of suitable bonding agents. These tightly linked the polymer to such solid ingredients as ammonium perchlorate and aluminum. Without such links, the propellant could not withstand the temperature cycling, ignition pressure, and other forces that could cause the solid particles to separate from the binder network. This would produce voids in the grain that could result in cracks and structural failure.
A key figure in the development of HTPB for use as a binder was Robert C. Corley, who served as a research chemist and project manager at the Air Force Rocket Propulsion Laboratory at Edwards AFB from 1966 to 1978 and rose through other positions to become the lab’s chief scientist from 1991 to 1997. But many other people from Thiokol, Aerojet, the army at Redstone Arsenal, Atlantic Research, Hercules, and the navy were also involved. Even HTPB did
not replace PBAN for all uses, including the Titan III, Titan IVA, and Space Shuttle solid-rocket motors, because PBAN could be produced for the comparatively low cost of $2.50 per pound at a rate in the 1980s of 4 million pounds per year, much higher than for any other propellant.69
To return to Minuteman II, however, the second major change in the stage-two motor was the shift to a single nozzle with liquid thrust vector control replacing movable nozzles for control in pitch and yaw. Static firings had shown that the same propellants produced seven to eight points less specific impulse when fired from four nozzles than from a single one. With the four nozzles, liquid particles agglomerated in their approach sections and produced exit-cone erosion, changing the configuration of the exit cone in 254 an unfavorable way. The solution was not only a single nozzle on Chapter 6 Minuteman II’s second stage but also the change in thrust vector control. The navy had begun testing a Freon system for thrust vector control in the second stage of Polaris A3 in September 1961, well before the Minuteman II, stage-two program began in February 1962. The system was low in weight, was insensitive to propellant flame temperature, and posed negligible constraints on the design of the nozzle. The Minuteman engineers adopted it—but one more example of borrowings back and forth between the Polaris and Minuteman projects despite the air force’s view of Polaris as a threat to its roles and missions.
Despite this pioneering work by the navy and its contractors, according to TRW historians, their firm still had to determine how much “vector capability" stage two of Minuteman II would require. TRW analyzed the amount of injectant that could be used before sloshing in the tank permitted the ingestion of air, and it determined the system performance requirements. Since Aerojet was involved in the development of the system for Polaris, probably its participation in this process was also important. In any event, the Minute – man team, like the navy, used Freon as the injectant, confining it in a rubber bladder inside a metal pressure vessel. Both TRW and Aerojet studied the propensity of the Freon to “migrate" through the bladder wall and become unavailable for its intended purpose. They found that only 25 of 262 pounds of Freon would escape, leaving enough to provide the necessary control in pitch and yaw. A separate solid-propellant gas generator provided roll control. In addition to these changes, stage two of Minuteman II increased in length from 159.2 inches for Minuteman I to 162.32 inches. The diameter increased from 44.3 to 52.17 inches, resulting in an overall weight increase from 11,558.9 to 15,506 pounds. Some 3,382.2 of this ad-
ditional 3,947.1 pounds consisted of propellant weight. Even so, the propellant mass fraction decreased slightly from 0.897 to 0.887.70
The Strategic Air Command put the first Minuteman II squadron on operational alert in May 1966, with initial operational capability declared as of December 1966. In the next few years, the air force began replacing Minuteman Is with Minuteman IIs.71
MINUTEMAN III
 Minuteman III featured multiple, independently targetable reentry vehicles with a liquid fourth stage for deployment of this payload. This last feature was not particularly relevant to launch-vehicle development except that the added weight required for it necessitated higher booster performance. Stages one and two did not change from Minuteman II, but stage three became larger. Hercules lost the contract for the larger motor to Aerojet. Subsequently, Thiokol and a new organization, the Chemical Systems Division of United Technologies Corporation, won contracts to build replacement motors. Stage three featured a fiberglass motor case, the same basic propellant Aerojet had used in stage two only in slightly different proportions, a single nozzle that was fixed in place and partially submerged into the case, a liquid-injection thrust-vector-control system for control in pitch and yaw, a separate roll-control system, and a thrust-termination system.
Minuteman III featured multiple, independently targetable reentry vehicles with a liquid fourth stage for deployment of this payload. This last feature was not particularly relevant to launch-vehicle development except that the added weight required for it necessitated higher booster performance. Stages one and two did not change from Minuteman II, but stage three became larger. Hercules lost the contract for the larger motor to Aerojet. Subsequently, Thiokol and a new organization, the Chemical Systems Division of United Technologies Corporation, won contracts to build replacement motors. Stage three featured a fiberglass motor case, the same basic propellant Aerojet had used in stage two only in slightly different proportions, a single nozzle that was fixed in place and partially submerged into the case, a liquid-injection thrust-vector-control system for control in pitch and yaw, a separate roll-control system, and a thrust-termination system.
Aerojet had moved its filament-wound case production to Sacramento. It produced most of the Minuteman fiberglass combustion chambers there but ceased winding filament in 1965. Meanwhile, Young had licensed his Spiralloy technology to Black, Sivalls, and Bryson in Oklahoma City, which became a second source for the Minuteman third-stage motor case. This instance and the three firms involved in producing the third stage illustrate the extent to which technology transferred among the contractors and subcontractors for government missiles and rockets.
The issue of technology transfer among competing contractors and the armed services, which were also competing over funds and missions, is a complex one about which a whole chapter—even a book—could be written. To address the subject briefly, there had been a degree of effort to exchange knowledge about rocket propulsion technology beginning in 1946 when the navy provided funding for a Rocket Propellant Information Agency (RPIA) within the Johns Hopkins University’s Applied Physics Laboratory. The army added support in 1948, and the RPIA became the Solid Propellant Information Agency (SPIA). The air force joined the other services in 1951. After the Sputnik launch, the newly created NASA be-
gan participating in SPIA activities in 1959. Meanwhile, the navy created the Liquid Propellant Information Agency (LIPA) in 1958. The SPIA and LPIA combined on December 1, 1962, to create the Chemical Propulsion Information Agency (CPIA).
With the further development of rockets and missiles, the need had become obvious by 1962 for a better exchange of information. So the DoD created an Interagency Chemical Rocket Propulsion Group in November 1962, the name later changing to the Joint Army/Navy/NASA/Air Force (JANNAF) Interagency Propulsion Committee. Together with CPIA, JANNAF effectively promoted sharing of technology. In addition, “joint-venture" contracts, pioneered by Levering Smith of the navy, often mandated the sharing of manufacturing technology among companies. These contracts 256 served to eliminate the services’ dependence for a given technology Chapter 6 on sole sources that could be destroyed by fire or possible enemy targeting. It also provided for competitive bidding on future contracts. The air force had a similar policy.72
Meanwhile, the propellant for Aerojet’s third stage of Minuteman had less CTPB and more aluminum than the second stage. The grain configuration consisted of an internal-burning cylindrical bore with six “fins" radiating out in the forward end. The igniter used black – powder squibs to start some of the CTPB propellant, which in turn spread the burning to the grain itself. The 50 percent submerged nozzle had a graphite phenolic entrance section, a forged tungsten throat insert, and a carbon-phenolic exit cone. As compared with the 85.25-inch-long, 37.88-inch-diameter third stage of Minuteman II, that for Minuteman III was 91.4 inches long and 52 inches in diameter. The mass fraction improved from 0.864 to 0.910, and with a nearly 10-lbf-sec/lbm greater specific impulse, the new third stage had more than twice the total impulse of its predecessor—2,074,774 as compared with 1,006,000 pounds force per second.73
The thrust-vector-control system for the new stage three was similar to that for stage two except that strontium perchlorate was used instead of Freon as the injectant into the thrust stream to provide control in the pitch and yaw axes. Helium gas provided the pressure to insert the strontium perchlorate instead of the solid – propellant gas generator used in the second stage. Roll control again came from a gas generator supplying gas to nozzles pointing in opposite directions. When both were operating, there was neutral torque in the roll axis. When roll torque was required, the flight – control system closed a flapper on one of the nozzles, providing unbalanced thrust to stop any incipient roll.
To ensure accuracy for the delivery of the warheads, Minute – man had always required precise thrust termination for stage three, determined by the flight-control computer. On Minuteman I, the thrust-termination system consisted of four thick carbon-phenolic tubes integrally wound in the sidewall of the third-stage case and sealed with snap-ring closures to form side ports. Detonation of explosive ordnance released a frangible section of the snap ring, thereby venting the combustion chamber and causing a momentary negative thrust that resulted in the third stage dropping away from the postboost vehicle.
 The system for Minuteman III involved six circular-shaped charges on the forward dome. Using data from high-speed films and strain gauges, the Minuteman team learned that this arrangement worked within 20 microseconds, cutting holes that resulted in a rupture of the pressure vessel within 2 additional milliseconds. But the case developed cracks radiating from the edge of the holes. TRW used a NASTRAN computer code to define propagation of the cracks. It then determined the dome thickness needed to eliminate the failure of the fiberglass. Aerojet wound “doilies" integrally into the dome of the motor case under each of the circular charges. This eliminated the rupturing, allowing the system to vent the pressure in the chamber and produce momentary negative thrust.74
The system for Minuteman III involved six circular-shaped charges on the forward dome. Using data from high-speed films and strain gauges, the Minuteman team learned that this arrangement worked within 20 microseconds, cutting holes that resulted in a rupture of the pressure vessel within 2 additional milliseconds. But the case developed cracks radiating from the edge of the holes. TRW used a NASTRAN computer code to define propagation of the cracks. It then determined the dome thickness needed to eliminate the failure of the fiberglass. Aerojet wound “doilies" integrally into the dome of the motor case under each of the circular charges. This eliminated the rupturing, allowing the system to vent the pressure in the chamber and produce momentary negative thrust.74
Minuteman Ills achieved their initial operational capability in June 1970, the first squadron of the upgraded missiles turned over to an operational wing at Minot AFB, North Dakota, in January 1971. By July 1975, there were 450 Minuteman Ils and 550 Minute – man Ills deployed at Strategic Air Command bases.75
 One major area of difference between missile and launch-vehicle development lay in the requirement for special safeguards on launch vehicles that propelled humans into space. Except for Juno I and Vanguard, which were short-lived, among the first U. S. space-launch vehicles were the Redstones and Atlases used in Project Mercury and the Atlases and Titan IIs used in Project Gemini to prepare for the Apollo Moon Program. Both Projects Mercury and Gemini required a process called “man-rating" (at a time before there were women serving as astronauts). This process resulted in adaptations of the Redstone, Atlas, and Titan II missiles to make them safer for the human beings carried in Mercury and Gemini capsules.
One major area of difference between missile and launch-vehicle development lay in the requirement for special safeguards on launch vehicles that propelled humans into space. Except for Juno I and Vanguard, which were short-lived, among the first U. S. space-launch vehicles were the Redstones and Atlases used in Project Mercury and the Atlases and Titan IIs used in Project Gemini to prepare for the Apollo Moon Program. Both Projects Mercury and Gemini required a process called “man-rating" (at a time before there were women serving as astronauts). This process resulted in adaptations of the Redstone, Atlas, and Titan II missiles to make them safer for the human beings carried in Mercury and Gemini capsules.![]()
 May 5, 1961, from Pad 5 at Cape Canaveral. (Photo courtesy of NASA)
May 5, 1961, from Pad 5 at Cape Canaveral. (Photo courtesy of NASA) For Titan II-Gemini, there were major problems with longitudinal oscillations in the engines, known as pogo (from their resemblance to the gyrations of the then-popular plaything, the pogo stick). These never occurred in flight but appeared in a severe form during static testing of second-stage engines. Surges in the oxidizer feed lines were causing the problem, which Martin engineers and others solved with suppression mechanisms. There was also the issue of combustion instability that occurred on only 2 percent of the ground tests of second-stage engines. But for man-rating, even this was too high. Aerojet (the Titan engine contractor) solved the problem with a new injector.6
For Titan II-Gemini, there were major problems with longitudinal oscillations in the engines, known as pogo (from their resemblance to the gyrations of the then-popular plaything, the pogo stick). These never occurred in flight but appeared in a severe form during static testing of second-stage engines. Surges in the oxidizer feed lines were causing the problem, which Martin engineers and others solved with suppression mechanisms. There was also the issue of combustion instability that occurred on only 2 percent of the ground tests of second-stage engines. But for man-rating, even this was too high. Aerojet (the Titan engine contractor) solved the problem with a new injector.6


 In addition to a malfunction detection system, features added to the Titan II missile for astronaut safety included redundant components of the electrical systems. To help compensate for all the weight from the additional components, engineers also deleted vernier and retro-rockets, which were not necessary for the Gemini mission.8 From March 23, 1965, to November 11, 1966, Gemini 3 through 12 all carried two astronauts on the Gemini spacecraft. These missions had their problems as well as their triumphs. But with them, the United States finally assumed the lead in the space race with its cold-war rival, the Soviet Union. Despite lots of problems, Gemini had prepared the way for the Apollo Moon landings and achieved its essential objectives.9
In addition to a malfunction detection system, features added to the Titan II missile for astronaut safety included redundant components of the electrical systems. To help compensate for all the weight from the additional components, engineers also deleted vernier and retro-rockets, which were not necessary for the Gemini mission.8 From March 23, 1965, to November 11, 1966, Gemini 3 through 12 all carried two astronauts on the Gemini spacecraft. These missions had their problems as well as their triumphs. But with them, the United States finally assumed the lead in the space race with its cold-war rival, the Soviet Union. Despite lots of problems, Gemini had prepared the way for the Apollo Moon landings and achieved its essential objectives.9










 Despite the problems with the second stage of Vanguard, the air force used a modified version on its Thor-Able launch vehicle, showing the transfer of technology from the navy to the air service. The Able was more successful than the Vanguard prototype for two reasons. One was special cleaning and handling techniques for the propellant tanks that came into being after Vanguard had taken delivery of many of its tanks. Also, Thor-Able did not need to extract maximum performance from the second stage as Vanguard did, so it did not have to burn the very last dregs of propellant in the tanks. The residue that the air force did not need to burn contained more of the scale from the tanks than did the rest of the propellant. Consequently, the valves could close before most of the scale entered the fuel lines, the evident cause of many of Vanguard’s problems.30
Despite the problems with the second stage of Vanguard, the air force used a modified version on its Thor-Able launch vehicle, showing the transfer of technology from the navy to the air service. The Able was more successful than the Vanguard prototype for two reasons. One was special cleaning and handling techniques for the propellant tanks that came into being after Vanguard had taken delivery of many of its tanks. Also, Thor-Able did not need to extract maximum performance from the second stage as Vanguard did, so it did not have to burn the very last dregs of propellant in the tanks. The residue that the air force did not need to burn contained more of the scale from the tanks than did the rest of the propellant. Consequently, the valves could close before most of the scale entered the fuel lines, the evident cause of many of Vanguard’s problems.30
 Long before the final launch of the Thor-Able, nevertheless, the Department of Defense’s Advanced Research Projects Agency had
Long before the final launch of the Thor-Able, nevertheless, the Department of Defense’s Advanced Research Projects Agency had Meanwhile, in the fall of 1959 Bell began designing the engine for the Agena D, which became the standard Agena propulsion unit. From June 28, 1962, to May 25, 1972, a large number of Thor-Agena D launches occurred, but because of the classified nature of many of the payloads, a reliable and precise tally is not available. During this period, the basic booster changed from the thrust-augmented to the long-tank, thrust-augmented Thor (called Thorad) with increased burning time and improved strap-on boosters.21
Meanwhile, in the fall of 1959 Bell began designing the engine for the Agena D, which became the standard Agena propulsion unit. From June 28, 1962, to May 25, 1972, a large number of Thor-Agena D launches occurred, but because of the classified nature of many of the payloads, a reliable and precise tally is not available. During this period, the basic booster changed from the thrust-augmented to the long-tank, thrust-augmented Thor (called Thorad) with increased burning time and improved strap-on boosters.21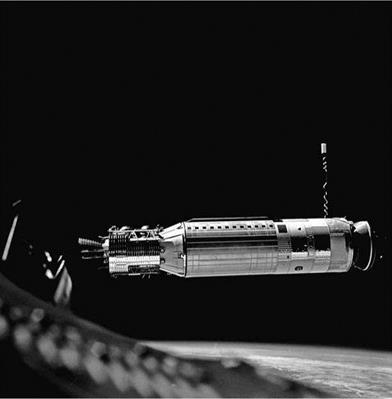 denberg AFB into sun-synchronous orbits, one of them a mission failure. The spacecraft were classified at the time but appear to have been Block 4A Defense Satellite Applications Program weather satellites used to inform the U. S. military of weather conditions for launching reconnaissance satellites and other defense purposes, such as mission planning during the conflict in Vietnam. In 1973, the program became the Defense Meteorological Satellite Program (DMSP) and was no longer classified.22
denberg AFB into sun-synchronous orbits, one of them a mission failure. The spacecraft were classified at the time but appear to have been Block 4A Defense Satellite Applications Program weather satellites used to inform the U. S. military of weather conditions for launching reconnaissance satellites and other defense purposes, such as mission planning during the conflict in Vietnam. In 1973, the program became the Defense Meteorological Satellite Program (DMSP) and was no longer classified.22 A final major Thor launch vehicle was the Thor-Delta. Whereas the other Thor-based launch vehicles were primarily air force assets sometimes used by NASA, Delta was a NASA-developed space – launch vehicle used on occasion by the air force until near the end of the period covered by this book, when the air force began to make extensive use of Delta IIs. Since it was conceived by NASA in 1959 as an interim vehicle to lift medium payloads by using existing technology, modified only as needed for specific missions, Delta has enjoyed a remarkably long career, attesting to its success.26
A final major Thor launch vehicle was the Thor-Delta. Whereas the other Thor-based launch vehicles were primarily air force assets sometimes used by NASA, Delta was a NASA-developed space – launch vehicle used on occasion by the air force until near the end of the period covered by this book, when the air force began to make extensive use of Delta IIs. Since it was conceived by NASA in 1959 as an interim vehicle to lift medium payloads by using existing technology, modified only as needed for specific missions, Delta has enjoyed a remarkably long career, attesting to its success.26

 Although there were other important upper-stage engines using storable propellants, such as the Bell Agena engine (starting with the 8048 model), the most significant engines with hypergolic propellants were those used in the Titans II, III, and IV, designed and built by Aerojet. Here, the previous experience with Vanguard, Able, and Able-Star undoubtedly were extraordinarily valuable, as was Aerojet’s involvement with the development of UDMH. In the latter development, Aerojet propellant chemist Karl Klager had been an important contributor. Klager held a Ph. D. in chemistry from the University of Vienna (1934) and had come to this country as part of Project Paperclip quite independently of the von Braun group. He worked for the Office of Naval Research in Pasadena during 1949 and started with Aerojet in 1950. The following year, Aerojet received a contract to develop an in-flight thrust-augmentation rocket for the F-86 fighter. The device never went into production, but in developing it, Aerojet engineers conducted a literature search for candidate propellants, did theoretical performance calculations, and measured physical and chemical properties in the laboratory. Together with RFNA, UDMH seemed highly promising but was not available in sufficient quantities to be used. Klager devised (and patented) production processes that yielded large quantities at reasonable prices, but workers began to get violently ill from the toxic substance. All did recover, and Aerojet learned how to control exposure to the vapors.37
Although there were other important upper-stage engines using storable propellants, such as the Bell Agena engine (starting with the 8048 model), the most significant engines with hypergolic propellants were those used in the Titans II, III, and IV, designed and built by Aerojet. Here, the previous experience with Vanguard, Able, and Able-Star undoubtedly were extraordinarily valuable, as was Aerojet’s involvement with the development of UDMH. In the latter development, Aerojet propellant chemist Karl Klager had been an important contributor. Klager held a Ph. D. in chemistry from the University of Vienna (1934) and had come to this country as part of Project Paperclip quite independently of the von Braun group. He worked for the Office of Naval Research in Pasadena during 1949 and started with Aerojet in 1950. The following year, Aerojet received a contract to develop an in-flight thrust-augmentation rocket for the F-86 fighter. The device never went into production, but in developing it, Aerojet engineers conducted a literature search for candidate propellants, did theoretical performance calculations, and measured physical and chemical properties in the laboratory. Together with RFNA, UDMH seemed highly promising but was not available in sufficient quantities to be used. Klager devised (and patented) production processes that yielded large quantities at reasonable prices, but workers began to get violently ill from the toxic substance. All did recover, and Aerojet learned how to control exposure to the vapors.37
 Agena and its payload ride into space aboard a large booster rocket. Following staging, the Agena engine "first-burn" maneuvers the vehicle and its payload into an earth – oriented parking orbit. The Agena "second-burn" is geared to each particular mission – for example, an elliptical earth orbit or the ejection of a payload on a trajectory to the moon or planets.
Agena and its payload ride into space aboard a large booster rocket. Following staging, the Agena engine "first-burn" maneuvers the vehicle and its payload into an earth – oriented parking orbit. The Agena "second-burn" is geared to each particular mission – for example, an elliptical earth orbit or the ejection of a payload on a trajectory to the moon or planets. In May 1960, the air force signed a letter contract with the Martin Company to develop, produce, and test the Titan II. On October 9, 1959, Aerojet had already won approval to convert the Titan I engines to burn storable propellants. Research and development to that end began in January 1960. The Aerojet engineers also worked to achieve the improved performance called for in the April 30, 1960, plan for Titan II. Although the Titan II engines were based on those for Titan I, the new propellants and the requirements in the April 30 plan necessitated considerable redesign. Because the modifications did not always work as anticipated, the engineers had to resort to empirical solutions until they found the combinations that worked correctly and provided the necessary performance. Since the propellants were hypergolic, there was no need for an igniter in the Titan II engines (called XLR87-AJ-5 for the two engines in stage one and XLR91-AJ-5 for the single stage-two engine). The injectors for the Titan I engines had used alternating fuel and oxidizer passages with oxidizer impinging on oxidizer and fuel on fuel (called like-on – like) to obtain the necessary mixing of the two propellants. For the Titan II, Aerojet engineers tried fuel-on-oxidizer impingement. This evidently mixed the droplets of propellant better because higher performance resulted. But the improvement led to erosion of the injector face, necessitating a return to the like-on-like pattern.
In May 1960, the air force signed a letter contract with the Martin Company to develop, produce, and test the Titan II. On October 9, 1959, Aerojet had already won approval to convert the Titan I engines to burn storable propellants. Research and development to that end began in January 1960. The Aerojet engineers also worked to achieve the improved performance called for in the April 30, 1960, plan for Titan II. Although the Titan II engines were based on those for Titan I, the new propellants and the requirements in the April 30 plan necessitated considerable redesign. Because the modifications did not always work as anticipated, the engineers had to resort to empirical solutions until they found the combinations that worked correctly and provided the necessary performance. Since the propellants were hypergolic, there was no need for an igniter in the Titan II engines (called XLR87-AJ-5 for the two engines in stage one and XLR91-AJ-5 for the single stage-two engine). The injectors for the Titan I engines had used alternating fuel and oxidizer passages with oxidizer impinging on oxidizer and fuel on fuel (called like-on – like) to obtain the necessary mixing of the two propellants. For the Titan II, Aerojet engineers tried fuel-on-oxidizer impingement. This evidently mixed the droplets of propellant better because higher performance resulted. But the improvement led to erosion of the injector face, necessitating a return to the like-on-like pattern.
 A 1965 Aerojet news release on Titan II propulsion credited it to “the efforts of hundreds of men and women" but singled out four of them as leaders of the effort. Three of their biographies illustrate the way that engineers in aerospace migrated from one firm to another or from government work to the private sector, carrying their knowledge of various technologies with them. Robert B. Young was the overall manager of the design, development, and production effort for both Titan I and Titan II. He was a chemical engineering graduate of Caltech, where Theodore von Karman had encouraged him to devote his knowledge to rocketry. He had worked for a year as director of industrial liaison on the Saturn program at NASA’s Marshall Space Flight Center, during part of the Titan years, and had risen within Aerojet’s own structure from a project engineer to a vice president and manager of the Sacramento, California, plant. Another leader was Ray C. Stiff Jr., who had discovered aniline as a hypergolic propellant while working at the navy Engineering Experiment Station in Annapolis. His role in the use of self-igniting propellants in JATOs to assist a PBY into the air while carrying heavy loads proved a forerunner “of the storable, self-igniting propellants used to such advantage in the Titan II engine systems." Stiff served as Aerojet’s manager of Liquid Rocket Operations near Sacramento and was also a vice president.
A 1965 Aerojet news release on Titan II propulsion credited it to “the efforts of hundreds of men and women" but singled out four of them as leaders of the effort. Three of their biographies illustrate the way that engineers in aerospace migrated from one firm to another or from government work to the private sector, carrying their knowledge of various technologies with them. Robert B. Young was the overall manager of the design, development, and production effort for both Titan I and Titan II. He was a chemical engineering graduate of Caltech, where Theodore von Karman had encouraged him to devote his knowledge to rocketry. He had worked for a year as director of industrial liaison on the Saturn program at NASA’s Marshall Space Flight Center, during part of the Titan years, and had risen within Aerojet’s own structure from a project engineer to a vice president and manager of the Sacramento, California, plant. Another leader was Ray C. Stiff Jr., who had discovered aniline as a hypergolic propellant while working at the navy Engineering Experiment Station in Annapolis. His role in the use of self-igniting propellants in JATOs to assist a PBY into the air while carrying heavy loads proved a forerunner “of the storable, self-igniting propellants used to such advantage in the Titan II engine systems." Stiff served as Aerojet’s manager of Liquid Rocket Operations near Sacramento and was also a vice president. Although the standpipe later proved to be part of the solution to the pogo problem, initially it seemed to make matters worse. Installed on missile N-11, flying on December 6, 1962, it failed to suppress severe oscillations that raised the gravitational effect from pogo alone to ±5 Gs. This lowered the chamber pressure to the point that instrumentation shut down the first-stage engine prematurely. The following mission, N-13, on December 19, 1962, did not include the standpipe but did have increased pressure in the fuel tank, which had seemed to be effective against the pogo effect on earlier flights. Another new feature consisted of aluminum oxidizer feed lines in place of steel ones used previously. For reasons not fully understood, the pogo level dropped and the flight was successful.
Although the standpipe later proved to be part of the solution to the pogo problem, initially it seemed to make matters worse. Installed on missile N-11, flying on December 6, 1962, it failed to suppress severe oscillations that raised the gravitational effect from pogo alone to ±5 Gs. This lowered the chamber pressure to the point that instrumentation shut down the first-stage engine prematurely. The following mission, N-13, on December 19, 1962, did not include the standpipe but did have increased pressure in the fuel tank, which had seemed to be effective against the pogo effect on earlier flights. Another new feature consisted of aluminum oxidizer feed lines in place of steel ones used previously. For reasons not fully understood, the pogo level dropped and the flight was successful. A final problem that occurred in flight testing and required reengineering involved the gas generator in the stage-two engine. This issue was a matter of concern to the Titan II Program Office at BSD, not just to SSD and NASA. Engineers and managers first became aware there was a problem on the second Titan II test flight (June 7, 1962) when telemetry showed that the second-stage engine had achieved only half of its normal thrust soon after engine start. When the tracking system lost its signal from the vehicle, the range safety officer caused cutoff of the fuel flow, making the reentry vehicle splash down well short of its target area. The data from telemetry on this flight were inadequate for the engineers to diagnose the problem. It took two more occurrences of gas-generator problems to provide enough data to understand what was happening. Particles were partially clogging the small openings in the gas generator’s injectors. This restricted propellant flow and resulted in loss of thrust. Technicians very thoroughly removed all foreign matter from components of the generators in a clean room before assembly, separated the generators from the engines in transport, and subjected the assemblies to blowdown by nitrogen before each test flight to ensure no foreign particles were present.
A final problem that occurred in flight testing and required reengineering involved the gas generator in the stage-two engine. This issue was a matter of concern to the Titan II Program Office at BSD, not just to SSD and NASA. Engineers and managers first became aware there was a problem on the second Titan II test flight (June 7, 1962) when telemetry showed that the second-stage engine had achieved only half of its normal thrust soon after engine start. When the tracking system lost its signal from the vehicle, the range safety officer caused cutoff of the fuel flow, making the reentry vehicle splash down well short of its target area. The data from telemetry on this flight were inadequate for the engineers to diagnose the problem. It took two more occurrences of gas-generator problems to provide enough data to understand what was happening. Particles were partially clogging the small openings in the gas generator’s injectors. This restricted propellant flow and resulted in loss of thrust. Technicians very thoroughly removed all foreign matter from components of the generators in a clean room before assembly, separated the generators from the engines in transport, and subjected the assemblies to blowdown by nitrogen before each test flight to ensure no foreign particles were present. Despite this preparatory work for Minuteman, the missile did not begin formal development until the air force secured final DoD approval in February 1958. Meanwhile, civilian engineers employed by the air force at the Non-Rotating Engine Branch at Wright-Patterson AFB had continued efforts to develop large, solid-propellant motors. Perhaps from sources at Aerojet, they learned about adding large quantities of aluminum to a solid propellant to increase its performance. They combined this with other information they had been gathering on solid propellants while Hall was still with the branch. Bill Fagan from the branch carried this information to Hall at the Ballistic Missile Division (as the WDD had become).52
Despite this preparatory work for Minuteman, the missile did not begin formal development until the air force secured final DoD approval in February 1958. Meanwhile, civilian engineers employed by the air force at the Non-Rotating Engine Branch at Wright-Patterson AFB had continued efforts to develop large, solid-propellant motors. Perhaps from sources at Aerojet, they learned about adding large quantities of aluminum to a solid propellant to increase its performance. They combined this with other information they had been gathering on solid propellants while Hall was still with the branch. Bill Fagan from the branch carried this information to Hall at the Ballistic Missile Division (as the WDD had become).52 Among many other difficulties, a major concern was launching from a silo. The first successful launch of the missile occurred in early February 1961, which Phillips referred to as “December 63rd" since the planned date had been in December 1960. It and two succeeding launches took place from a surface pad and were so successful that the team advanced the first silo launch to August 1961. The missile blew up in the silo, giving credence to critics in STL who had argued that firing a missile from a silo was impossible. As Phillips said, the missile “really came out of there like a Roman candle."57
Among many other difficulties, a major concern was launching from a silo. The first successful launch of the missile occurred in early February 1961, which Phillips referred to as “December 63rd" since the planned date had been in December 1960. It and two succeeding launches took place from a surface pad and were so successful that the team advanced the first silo launch to August 1961. The missile blew up in the silo, giving credence to critics in STL who had argued that firing a missile from a silo was impossible. As Phillips said, the missile “really came out of there like a Roman candle."57 For stage two of Minuteman I, Aerojet used the polyurethane binder employed in Polaris, with ammonium perchlorate as the oxidizer and aluminum powder the major fuel. It used two slightly different propellant grains, with a faster-burning inner grain and a slower-burning outer one. The combination resulted in a conversion of the four-point, star-shaped, internal-burning cavity to a cylindrical one as the propellant burned, avoiding slivers of propellant that did not burn. The propellant yielded a vacuum specific impulse of nearly 275 lbf-sec/lbm at temperatures ranging from 60°F to 80°F.62
For stage two of Minuteman I, Aerojet used the polyurethane binder employed in Polaris, with ammonium perchlorate as the oxidizer and aluminum powder the major fuel. It used two slightly different propellant grains, with a faster-burning inner grain and a slower-burning outer one. The combination resulted in a conversion of the four-point, star-shaped, internal-burning cavity to a cylindrical one as the propellant burned, avoiding slivers of propellant that did not burn. The propellant yielded a vacuum specific impulse of nearly 275 lbf-sec/lbm at temperatures ranging from 60°F to 80°F.62 MINUTEMAN II
MINUTEMAN II It frequently happens in the history of technology that innovations occur to different people at about the same time. This appears to have been the case with CTPB, which Aerojet historians attribute to Phillips Petroleum and Rocketdyne without providing details. These two companies may have been the source for information about the CTPB that Aerojet used in Minuteman II, stage two. Like Thiokol, in any event, Aerojet proposed to use MAPO as a cross-linking agent. TRW historians state that their firm’s laboratory investigations revealed the hydrolysis problem. They state that “working with Aerojet’s research and development staff," they developed “a formulation that eliminated MAPO. . . ." The CTPB that resulted from what apparently was a multicompany development effort had better fuel values than previous propellants, good mechanical properties such as the long shelf life required for silo – based missiles, and a higher solids content than previous binders. The propellant consisted primarily of CTPB, ammonium perchlorate, and aluminum. It yielded a vacuum specific impulse more than 15 lbf-sec/lbm higher than the propellant used in stage two of Minuteman I, Wing II.68
It frequently happens in the history of technology that innovations occur to different people at about the same time. This appears to have been the case with CTPB, which Aerojet historians attribute to Phillips Petroleum and Rocketdyne without providing details. These two companies may have been the source for information about the CTPB that Aerojet used in Minuteman II, stage two. Like Thiokol, in any event, Aerojet proposed to use MAPO as a cross-linking agent. TRW historians state that their firm’s laboratory investigations revealed the hydrolysis problem. They state that “working with Aerojet’s research and development staff," they developed “a formulation that eliminated MAPO. . . ." The CTPB that resulted from what apparently was a multicompany development effort had better fuel values than previous propellants, good mechanical properties such as the long shelf life required for silo – based missiles, and a higher solids content than previous binders. The propellant consisted primarily of CTPB, ammonium perchlorate, and aluminum. It yielded a vacuum specific impulse more than 15 lbf-sec/lbm higher than the propellant used in stage two of Minuteman I, Wing II.68 Minuteman III featured multiple, independently targetable reentry vehicles with a liquid fourth stage for deployment of this payload. This last feature was not particularly relevant to launch-vehicle development except that the added weight required for it necessitated higher booster performance. Stages one and two did not change from Minuteman II, but stage three became larger. Hercules lost the contract for the larger motor to Aerojet. Subsequently, Thiokol and a new organization, the Chemical Systems Division of United Technologies Corporation, won contracts to build replacement motors. Stage three featured a fiberglass motor case, the same basic propellant Aerojet had used in stage two only in slightly different proportions, a single nozzle that was fixed in place and partially submerged into the case, a liquid-injection thrust-vector-control system for control in pitch and yaw, a separate roll-control system, and a thrust-termination system.
Minuteman III featured multiple, independently targetable reentry vehicles with a liquid fourth stage for deployment of this payload. This last feature was not particularly relevant to launch-vehicle development except that the added weight required for it necessitated higher booster performance. Stages one and two did not change from Minuteman II, but stage three became larger. Hercules lost the contract for the larger motor to Aerojet. Subsequently, Thiokol and a new organization, the Chemical Systems Division of United Technologies Corporation, won contracts to build replacement motors. Stage three featured a fiberglass motor case, the same basic propellant Aerojet had used in stage two only in slightly different proportions, a single nozzle that was fixed in place and partially submerged into the case, a liquid-injection thrust-vector-control system for control in pitch and yaw, a separate roll-control system, and a thrust-termination system. The system for Minuteman III involved six circular-shaped charges on the forward dome. Using data from high-speed films and strain gauges, the Minuteman team learned that this arrangement worked within 20 microseconds, cutting holes that resulted in a rupture of the pressure vessel within 2 additional milliseconds. But the case developed cracks radiating from the edge of the holes. TRW used a NASTRAN computer code to define propagation of the cracks. It then determined the dome thickness needed to eliminate the failure of the fiberglass. Aerojet wound “doilies" integrally into the dome of the motor case under each of the circular charges. This eliminated the rupturing, allowing the system to vent the pressure in the chamber and produce momentary negative thrust.74
The system for Minuteman III involved six circular-shaped charges on the forward dome. Using data from high-speed films and strain gauges, the Minuteman team learned that this arrangement worked within 20 microseconds, cutting holes that resulted in a rupture of the pressure vessel within 2 additional milliseconds. But the case developed cracks radiating from the edge of the holes. TRW used a NASTRAN computer code to define propagation of the cracks. It then determined the dome thickness needed to eliminate the failure of the fiberglass. Aerojet wound “doilies" integrally into the dome of the motor case under each of the circular charges. This eliminated the rupturing, allowing the system to vent the pressure in the chamber and produce momentary negative thrust.74 The intellectual push for Centaur came from Convair Division of General Dynamics engineer Krafft Ehricke, who had worked for von Braun at Peenemunde and Huntsville and for Bell Aircraft before moving to Convair. When General Dynamics managers asked him to design an upper stage for Atlas, he and some other engineers, including Bossart, decided that liquid hydrogen and liquid oxygen were the propellants they needed. Aware to some degree that liquid hydrogen’s very low density, extremely cold boiling point (-423°F), low surface tension, and wide range of flammability made it unusually difficult to work with, Ehricke faced funding limitations under an air force contract that precluded performing as many tests as the propellant required—an important restriction on normal rocketengineering practice.38
The intellectual push for Centaur came from Convair Division of General Dynamics engineer Krafft Ehricke, who had worked for von Braun at Peenemunde and Huntsville and for Bell Aircraft before moving to Convair. When General Dynamics managers asked him to design an upper stage for Atlas, he and some other engineers, including Bossart, decided that liquid hydrogen and liquid oxygen were the propellants they needed. Aware to some degree that liquid hydrogen’s very low density, extremely cold boiling point (-423°F), low surface tension, and wide range of flammability made it unusually difficult to work with, Ehricke faced funding limitations under an air force contract that precluded performing as many tests as the propellant required—an important restriction on normal rocketengineering practice.38
 The first use of the Centaur D-1A and SLV-3D was on the launch of Pioneer 11, which had the same mission as Pioneer 10 plus making detailed observations of Saturn and its rings. As on Pioneer 10, the mission also employed the Star 37 motor in a third stage. Launched on April 5, 1973, Pioneer 11 returned much data about Saturn, including discoveries of Saturn’s 11th moon and two new rings. Between 1973 and May 19, 1983, 32 SLV-3Ds launched with Centaur
The first use of the Centaur D-1A and SLV-3D was on the launch of Pioneer 11, which had the same mission as Pioneer 10 plus making detailed observations of Saturn and its rings. As on Pioneer 10, the mission also employed the Star 37 motor in a third stage. Launched on April 5, 1973, Pioneer 11 returned much data about Saturn, including discoveries of Saturn’s 11th moon and two new rings. Between 1973 and May 19, 1983, 32 SLV-3Ds launched with Centaur Partway through the history of Centaur and the various Atlas models used to launch it, the air force contracted with General Dynamics, beginning on February 14, 1966, to modify Atlas Es and Fs that had been in storage since their decommissioning as missiles in 1965. The process began with the newer F models. Rocketdyne inspected each of the MA-3 engines and fixed or replaced any part that failed to meet specifications. In 1969, the rocket division started a more extensive program of refurbishment to ensure that the engines in storage would work when called upon. After two launch failures in 1980-81, Rocketdyne rebuilt the engines at its plant, performing static tests before installing them on a launch vehicle.47
Partway through the history of Centaur and the various Atlas models used to launch it, the air force contracted with General Dynamics, beginning on February 14, 1966, to modify Atlas Es and Fs that had been in storage since their decommissioning as missiles in 1965. The process began with the newer F models. Rocketdyne inspected each of the MA-3 engines and fixed or replaced any part that failed to meet specifications. In 1969, the rocket division started a more extensive program of refurbishment to ensure that the engines in storage would work when called upon. After two launch failures in 1980-81, Rocketdyne rebuilt the engines at its plant, performing static tests before installing them on a launch vehicle.47 Conceived as a missile, the Atlas became a successful and versatile launch vehicle, mated with a great variety of upper stages. Featuring a controversial but “brilliant, innovative, and yet simple" concept (the steel-balloon tank design), both the Atlas and the Centaur proved to be flexible and effective. With commercialization, the Atlas and the Centaur continued to provide launch-vehicle services beyond the period of this book and into the 21st century. The Centaur proved to be especially difficult to develop because of the peculiar properties of liquid hydrogen. But it was also hampered by initial funding arrangements and other avoidable problems. As with many rocket programs, engineers found that the existing fund of knowledge was inadequate to predict all of the problems that would occur in developing and launching an extraordinarily complex machine. Unforeseen problems continued into the 1990s, and engineers had to relearn the lesson that continual and sophisticated testing was the price of success, even if it did not always preclude unanticipated failures.54
Conceived as a missile, the Atlas became a successful and versatile launch vehicle, mated with a great variety of upper stages. Featuring a controversial but “brilliant, innovative, and yet simple" concept (the steel-balloon tank design), both the Atlas and the Centaur proved to be flexible and effective. With commercialization, the Atlas and the Centaur continued to provide launch-vehicle services beyond the period of this book and into the 21st century. The Centaur proved to be especially difficult to develop because of the peculiar properties of liquid hydrogen. But it was also hampered by initial funding arrangements and other avoidable problems. As with many rocket programs, engineers found that the existing fund of knowledge was inadequate to predict all of the problems that would occur in developing and launching an extraordinarily complex machine. Unforeseen problems continued into the 1990s, and engineers had to relearn the lesson that continual and sophisticated testing was the price of success, even if it did not always preclude unanticipated failures.54 On July 23, 1963, Aerojet had successfully operated a Transtage engine for 4 minutes, 44 seconds, considered “a long duration firing." During that static test, the engine started and stopped three times, demonstrating the restart capability. However, a more critical test of this crucial capability (which would allow it to place multiple satellites into different orbits on a single launch or to position a single satellite in a final orbit, such as a geostationary orbit, without a need for a separate kick motor) would occur in the simulated-altitude test chamber at Arnold Engineering Development Center in Tullahoma, Tennessee. In August 1963, tests at that center confirmed suspicions from the July 23 test that the combustion chamber would burn through before completing a full-duration firing (undefined). In addition, gimballing of the engine in a cold environment revealed a malfunction of a bipropellant valve (that fed propellants to the combustion chamber) and a weakness in the nozzle extension, made of aluminide-coated columbium and radiation cooled with an expansion ratio of 40:1. Information about how Aerojet solved these problems is not available in any of the sources for this book, with the official history of the Titan III merely stating that “by the close of 1963, an extensive redesign and testing program was underway to eliminate these difficulties so the contractor could make his first delivery of flight engine hardware—due in mid – December 1963." 55
On July 23, 1963, Aerojet had successfully operated a Transtage engine for 4 minutes, 44 seconds, considered “a long duration firing." During that static test, the engine started and stopped three times, demonstrating the restart capability. However, a more critical test of this crucial capability (which would allow it to place multiple satellites into different orbits on a single launch or to position a single satellite in a final orbit, such as a geostationary orbit, without a need for a separate kick motor) would occur in the simulated-altitude test chamber at Arnold Engineering Development Center in Tullahoma, Tennessee. In August 1963, tests at that center confirmed suspicions from the July 23 test that the combustion chamber would burn through before completing a full-duration firing (undefined). In addition, gimballing of the engine in a cold environment revealed a malfunction of a bipropellant valve (that fed propellants to the combustion chamber) and a weakness in the nozzle extension, made of aluminide-coated columbium and radiation cooled with an expansion ratio of 40:1. Information about how Aerojet solved these problems is not available in any of the sources for this book, with the official history of the Titan III merely stating that “by the close of 1963, an extensive redesign and testing program was underway to eliminate these difficulties so the contractor could make his first delivery of flight engine hardware—due in mid – December 1963." 55
 The nozzle was partially submerged, and for gimballing, it used the Flexseal design Thiokol had scaled up in the 156-inch motor testing from the Lockheed’s Lockseal design. It was capable of eight degrees of deflection, necessitated among other reasons by the shuttle’s now-familiar roll soon after liftoff to achieve its proper trajectory. Having less thrust, the space shuttle main engines were incapable of achieving the necessary amount of roll, and the liquid-injection thrust-vector-control system used on the Titan solid – rocket motors would not have met the more demanding requirements of the shuttle. Hence the importance of the Lockseal-Flexseal development during the Large Segmented Solid Rocket Motor Program supported by both NASA and the air force.28
The nozzle was partially submerged, and for gimballing, it used the Flexseal design Thiokol had scaled up in the 156-inch motor testing from the Lockheed’s Lockseal design. It was capable of eight degrees of deflection, necessitated among other reasons by the shuttle’s now-familiar roll soon after liftoff to achieve its proper trajectory. Having less thrust, the space shuttle main engines were incapable of achieving the necessary amount of roll, and the liquid-injection thrust-vector-control system used on the Titan solid – rocket motors would not have met the more demanding requirements of the shuttle. Hence the importance of the Lockseal-Flexseal development during the Large Segmented Solid Rocket Motor Program supported by both NASA and the air force.28
 Even early in launch-vehicle history, some missile programs had already begun to influence solid-propellant developments. In 1956, a creative group of engineers at Langley’s Pilotless Aircraft Research Division (PARD) began formulating ideas that led to the Scout launch vehicles. This group included Maxime A. Faget, later famous for designing spacecraft; Joseph G. Thibodaux Jr., who promoted the spherical design of some rocket and spacecraft motors beginning in 1955; Robert O. Piland, who put together the first multistage rocket to reach the speed of Mach 10; and William E. Stoney Jr., who became the first head of the group responsible for developing the Scout, which he also christened. Wallops, established as a test base for the National Advisory Committee for Aeronautics’ (NACA) Langley Memorial Aeronautical Laboratory in 1945, had a history of using rockets, individually or in stages, to gather data at high speeds on both aircraft models and rocket nose cones. These data made it possible to design supersonic aircraft and hypersonic missiles at a time when ground facilities were not yet capable of providing comparable information. It was a natural step for engineers working in such a program to conceive a multistage, hypersonic, solid-propellant rocket that could reach orbital speeds of Mach 18.32
Even early in launch-vehicle history, some missile programs had already begun to influence solid-propellant developments. In 1956, a creative group of engineers at Langley’s Pilotless Aircraft Research Division (PARD) began formulating ideas that led to the Scout launch vehicles. This group included Maxime A. Faget, later famous for designing spacecraft; Joseph G. Thibodaux Jr., who promoted the spherical design of some rocket and spacecraft motors beginning in 1955; Robert O. Piland, who put together the first multistage rocket to reach the speed of Mach 10; and William E. Stoney Jr., who became the first head of the group responsible for developing the Scout, which he also christened. Wallops, established as a test base for the National Advisory Committee for Aeronautics’ (NACA) Langley Memorial Aeronautical Laboratory in 1945, had a history of using rockets, individually or in stages, to gather data at high speeds on both aircraft models and rocket nose cones. These data made it possible to design supersonic aircraft and hypersonic missiles at a time when ground facilities were not yet capable of providing comparable information. It was a natural step for engineers working in such a program to conceive a multistage, hypersonic, solid-propellant rocket that could reach orbital speeds of Mach 18.32