Early Castable Composite Propellants
Castable composite propellants grew out of a grant Theodore von Karman and Frank Malina arranged with the National Academy of Sciences (NAS) Committee on Army Air Corps Research in January 1939. With the $1,000 allotment, Malina and
his associates at GALCIT studied jet-assisted takeoff (JATO) of aircraft and prepared a proposal for research on the subject. This led to an NAS contract for $10,000, effective July 1, 1939, and to subsequent contracts with the army air corps and the navy for JATO units (with both liquid and solid propellants). JATOs actually used rocket (rather than jet) thrust to help heavily loaded aircraft take off on a short runway.1
 The key individual in the development of the first castable composite propellant was John W. Parsons, a propellant chemist on Ma – lina’s team. Parsons, largely self-taught, had taken some chemistry courses from the University of Southern California in 1935-36 but did not graduate. He worked as a chemist for Hercules Powder Company in Los Angeles from 1932 to 1934 and then was chief chemist for Halifax Explosives Company in Saugas, California, from 1934 to 1938. In 1939-40, Parsons sought a solution to the problem of controlled burning for many seconds in a solid-propellant rocket motor. This was critical to the development of a JATO unit. It was he, apparently, who conceived the concept of “cigarette-burning" at only one end of the propellant. But repeated tests of powder, compressed into a chamber and coated with a variety of substances to form a seal with the chamber wall, resulted in explosions. Authorities von Karman consulted advised that a powder rocket could burn for only two or three seconds.
The key individual in the development of the first castable composite propellant was John W. Parsons, a propellant chemist on Ma – lina’s team. Parsons, largely self-taught, had taken some chemistry courses from the University of Southern California in 1935-36 but did not graduate. He worked as a chemist for Hercules Powder Company in Los Angeles from 1932 to 1934 and then was chief chemist for Halifax Explosives Company in Saugas, California, from 1934 to 1938. In 1939-40, Parsons sought a solution to the problem of controlled burning for many seconds in a solid-propellant rocket motor. This was critical to the development of a JATO unit. It was he, apparently, who conceived the concept of “cigarette-burning" at only one end of the propellant. But repeated tests of powder, compressed into a chamber and coated with a variety of substances to form a seal with the chamber wall, resulted in explosions. Authorities von Karman consulted advised that a powder rocket could burn for only two or three seconds.
Not satisfied with this expert opinion, von Karman characteristically turned to theory for a solution. He devised four differential equations describing the operation of the rocket motor and handed them to Malina for solution. In solving them, Malina discovered that, theoretically, if the combustion chamber were completely filled by the propellant charge, if the physical properties of the propellant and the ratio of the area of burning propellant to the throat area of the chamber’s nozzle remained constant, thrust also would do likewise and there would be no explosions. Encouraged by these findings, Parsons and others came up with a compressed powder design that worked effectively (after one initial explosion) for 152 successive motors used in successful flight tests of JATO units on an Ercoupe aircraft in August 1941, convincing the navy to contract for a variety of assisted takeoff motors.
After storage under varying temperatures, however, the motors usually exploded. Parsons then found a solution to that problem. Apparently watching a roofing operation about June 1942, he concluded that asphalt as a binder and fuel mixed with potassium perchlorate as an oxidizer would yield a stable propellant. This proved to be true. Thus the theory of von Karman and Malina combined with
 |
|
the practical knowledge and imagination of Parsons to produce a castable, composite solid propellant that, with later improvements, made large solid-propellant rockets possible. A fundamental technological breakthrough, this formed the basis of many later castable propellants with much higher performance than asphalt-potassium perchlorate.2
Meanwhile, to produce its JATOs (both solid and liquid), five members of the GALCIT project (Malina, von Karman, Martin Summerfield, Parsons, and Edward S. Forman), plus von Karman’s lawyer, Andrew G. Haley, formed the Aerojet Engineering Corporation in March 1942 (Aerojet General Corporation after its acquisition by General Tire and Rubber Company in 1944-45). Aerojet did much business with the army air forces and navy for JATO units during the war and became by 1950 the largest rocket engine manufacturer in the world and a leader in research and development of rocket technology. Until the acquisition by General Tire, Aerojet and the GALCIT project maintained close technical relations.3
The initial asphalt-potassium perchlorate propellant—known as GALCIT 53—did not have a particularly impressive performance
compared, for example, with ballistite (a double-base composition). But it operated effectively at temperatures down to 40°F. At even lower temperatures, however, GALCIT 53 cracked. It also melted in the tropical sun and was very smoky when burning. This last characteristic restricted visibility for the takeoff of second and follow-on aircraft using JATO units on a single runway. Consequently, researchers at GALCIT and its successor, JPL, began searching for an elastic binder with storage limits beyond GALCIT 53’s extremes of -9°F to 120°F. In particular, a young engineer named Charles Bartley, who was employed at JPL from June 1944 to August 1951, began examining synthetic rubbers and polymers, eventually hitting upon a liquid polysulfide compound designated LP-2 as a solid-propellant binder. The Thiokol Chemical Corporation made it for sealing aircraft tanks and other applications.4
 Like many innovations, LP-2 had resulted from an initial, inadvertent discovery. In 1926, Joseph C. Patrick, a physician who found chemistry more interesting than medicine, sought an inexpensive way to produce antifreeze from ethylene using sodium polysulfide as a hydrolyzing agent. His procedure yielded a synthetic rubber instead of antifreeze. It led him to cofound Thiokol, which marketed the material in the form of gaskets, sealants, adhesives, and coatings (the polysulfide polymer being resistant to weather, solvents, and electrical arcing). Then in 1942, Patrick and an employee, H. L. Ferguson, found a way to make the first liquid polymer that included no volatile solvent yet could be cured to form a rubberlike solid. During World War II it was used to seal fuel tanks in aircraft, gun turrets, fuselages, air ducts, and the like.5
Like many innovations, LP-2 had resulted from an initial, inadvertent discovery. In 1926, Joseph C. Patrick, a physician who found chemistry more interesting than medicine, sought an inexpensive way to produce antifreeze from ethylene using sodium polysulfide as a hydrolyzing agent. His procedure yielded a synthetic rubber instead of antifreeze. It led him to cofound Thiokol, which marketed the material in the form of gaskets, sealants, adhesives, and coatings (the polysulfide polymer being resistant to weather, solvents, and electrical arcing). Then in 1942, Patrick and an employee, H. L. Ferguson, found a way to make the first liquid polymer that included no volatile solvent yet could be cured to form a rubberlike solid. During World War II it was used to seal fuel tanks in aircraft, gun turrets, fuselages, air ducts, and the like.5
Before learning of LP-2, Bartley and his associates at JPL had tried a variety of moldable synthetic rubbers as both binders and fuels, including Buna-S, Buna-N, and neoprene. Neoprene had the best properties for use as a binder and burned the best of the lot, but molding it required high pressures. Like the extrusion process used with double-base propellants (forcing them through a die), this made the production of large propellant grains (masses of propellant) impractical. Meanwhile, Thiokol chemists had begun to release data about LP-2. At a meeting of the American Chemical Society, Bartley asked about a liquid that would polymerize to a solid elastomer (rubberlike substance). Frank M. McMillan, who represented Shell Oil in the San Francisco area, knew about Thiokol’s product and shared the information with Bartley, who acquired small quantities from Walt Boswell, Thiokol’s representative for the western United States.6
With encouragement from Army Ordnance and the navy, Bartley—joined by John I. Shafer, a JPL design engineer, and
H. Lawrence Thackwell Jr., a specialist in aircraft structures—began in 1947 to develop a small rocket designated Thunderbird, with a 6-inch diameter. They used it for testing whether polysulfide propellants could withstand the forces of high acceleration that a potential large launch vehicle might encounter. Bartley had already found that an end-burning grain of polysulfide propellant did not produce steady thrust but burned faster at first and then leveled off. He attributed this to accelerated burning along the case to form a convex cone, a hypothesis he confirmed by quenching the flame partway through the burn.
To solve the problem of unsteady thrust, the three JPL engineers adopted a grain design that had been developed in Great Britain in the late 1930s but was similar to one developed independently in 226 1946 for double-base propellants by an American, Edward W. Price.
Chapter 6 It featured an internal-burning, star-shaped cavity. This design protected the case from excess heat because the burning was in the middle of the propellant grain. It also provided a constant level of thrust because as the star points burned away, the internal cavity became a cylinder with roughly the same surface area as the initial star. Bartley had read about the star design from a British report he did not specify and had instructed Shafer to investigate it. Shafer found that the government-owned, contractor-operated Allegany Ballistics Laboratory had used the British design in the uncompleted Vicar rocket and a scaled-down version named the Curate. Using equations from the ABL report on the projects, Shafer began developing a number of star designs in 1947. Combining a polysulfide propellant with the star design and casting it in the case so that it bonded thereto, the team under Bartley produced the successful Thunderbird rocket that passed its flight tests in 1947-48.7
Another significant development was the replacement of potassium perchlorate as an oxidizer by ammonium perchlorate, which offered higher performance (specific impulse) and less smoke. Apparently, the Thunderbird used a propellant designated JPL 100, which contained a mixture of ammonium perchlorate and potassium perchlorate in a polysulfide binder. In 1947, however, JPL had developed a JPL 118 propellant that used only ammonium perchlorate as an oxidizer together with polysulfide as the binder and a couple of curing agents. Although this propellant had yet to be fully investigated in 1947, by mid-1948 JPL had tested it and showed that it had a specific impulse of at least 198 lbf-sec/lbm at sea level, using an expansion ratio of 10 for the rocket nozzle. This was still relatively low compared with a typical performance of double-base
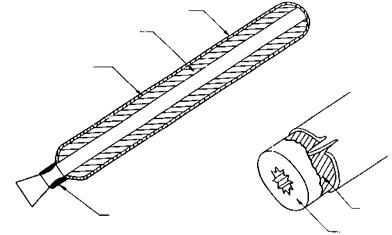 PROPELLANT
PROPELLANT
![]()
 |
 |
RUBBER-BASE LINER OR RESTRICTION BONDED TO CHAMBER
About this time, under contract to the army, Thiokol produced a T-40 motor intended for use as a JATO unit. As a propellant, it used JPL 100 (rechristened T-10 by Thiokol) in a case-bonded motor design. Also in 1949, Thiokol designed the T-41 motor for the Hughes Aircraft Company’s Falcon missile under development for the air force. This was a shorter version of JPL’s Thunderbird motor. It began production at Elkton, then moved to Huntsville, where a larger version called the T-42 evolved from it.11
According to Edward N. Hall, later an air force colonel who was important in promoting the development of solid propellants, the Falcon tactical (air-to-air) missile contributed “quality control techniques for rubber-base propellants, design data for case-bonded 228 grains, [and] aging characteristics of rubber-based propellants" to Chapter 6 the evolving store of knowledge about solid-propellant technology.
It appears that Thiokol did not make these contributions on its own. JPL provided considerable assistance in an early example of technology transfer. In October 1947, Charles Bartley of JPL was present at a meeting of Thiokol personnel, representatives of Army Ordnance, and the navy’s Bureau of Aeronautics to discuss the kind of work Thiokol was expected to do in the further development of polysulfide propellants. The next day, Bartley met again with Thiokol personnel to relate JPL’s experience with polysulfide-perchlorate propellants.12
In about January or February 1949, a trip report by a Thiokol employee discussed a visit to JPL’s Solid Rocket Section, of which Bartley was the chief. The report covered such matters as the grease used for extracting the mandrel to create the internal cavity in the grain once it cured and igniters that employed black or a special igniter powder. Also discussed were grinding ammonium and potassium perchlorate, combining them with the liquid polymer in a vertical mixer, pouring the propellant, preparing the liner for the combustion chamber, and testing. Also reported was a visit to Western Electrochemical Company, which supplied the perchlorate. The document concluded with some recommended changes in Thiokol’s operating procedures at Elkton.13
As helpful as JPL’s assistance was, however, the contributions Hall mentions seem to have come also from work done independently at Thiokol’s Elkton and Huntsville plants. For example, Thiokol discovered that the size of perchlorate particles was important in motor operation and propellant castability, so it introduced a micromerograph to measure particle size. To reduce the deleterious absorption of moisture by the perchlorates, Thiokol installed
air conditioning in the grinding rooms. The firm determined the optimal mixing time for the propellant and replaced a barium grease JPL had used to extract the mandrel from the middle of the cast propellant after curing with a Teflon coating. This latter step was necessary not only because the grease-affected part of the propellant had to be sanded after extraction but also because the grease had enfolded into the propellant, causing weak areas. Thiokol also introduced a “temperature-programmed cure cycle," pressurized curing, and a method of casting that eliminated propellant voids resulting from shrinkage and air bubbles.14 These details provide early examples of information—rare in the literature about rocketry—about which firms introduced specific innovations, illustrating the ways technology sometimes transferred.










