Working with the Palette of Light
We inhabit a physical universe filled with electromagnetic radiation spanning a factor of trillions in wavelength. The human eye sees only a sliver of this radiation, designated as visible light. It’s as if there was a full piano keyboard in front of us with eighty-eight keys and we were restricted to making music with two adjacent keys or notes. Space telescopes like Spitzer and the Chandra X – ray Observatory, covered in the next chapter, have opened up the palette of sensation and given us an octave or more with which to view the universe. Infrared astronomy, in particular, has been instrumental in revealing how stars and their planetary systems form. Spitzer can see through the vast clouds of dust, within which myriad stars are being born, and can penetrate the granular torus or dense disk that typically surround newborn stars. This capability has literally opened people’s eyes to the worlds that lie beyond the red end of the rainbow.
Isaac Newton first identified the colors of the visible spectrum by observing sunlight passed through a glass prism. William Her – schel later investigated temperatures associated with each color of visible light. Having in 1781 discovered Uranus, the first new planet since antiquity, Herschel was already the greatest astronomer of his age when he began experimenting with light. He noticed that heat passed through various colored filters used to observe the Sun and carried out experiments to understand this phenomenon. Using a thermometer with a soot-blackened bulb to better absorb heat, Herschel measured the temperature of each color band of visible light and noticed that the temperature increased from the violet to the red end of the visible spectrum.6 Surprised to record the highest temperatures beyond the edge of the red band, Herschel realized that electromagnetic radiation existed in wavelengths we can’t see. He had detected infrared light. Such early discoveries regarding the electromagnetic spectrum paved the way for remarkable new possibilities in studying the universe.
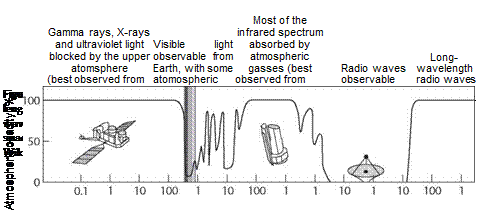
Light is conventionally subdivided by wavelength into radio, microwave, infrared, visible, ultraviolet, X-ray, and gamma ray, with radio designating cool, long wavelengths and gamma rays representing the highest energy, short wavelengths. These subdivisions are merely convenient demarcations for differences along a continuum of electromagnetic radiation. While most forms of radiation can’t reach the ground from sources in space (figure 9.1), all forms of electromagnetic radiation, from radio to gamma rays, travel at the same speed as light in a vacuum, approximately 300,000 kilometers per second or 186,000 miles per second. We’re intimately familiar with most of these bands of radiation. Radio waves transmit music and news on your favorite radio station. Microwaves make possible communication technologies including cell phones and, of course, in microwave ovens. Ultraviolet radiation can quickly give you a suntan or sunburn and is the means by
 |
which our bodies produce Vitamin D. We became familiar with UV light from the 1970s black lights. X-rays have been invaluable in medical and dental practice as well as in fluoroscopy, which offers real-time views through living tissue, while high-energy lasers are used in brain and eye surgery and other delicate medical procedures. Radiation at infrared wavelengths also has powerful therapeutic effects. Low-level, “cold laser” therapy contributes to the healing of wounds as noted in a report cited by the National Center for Biotechnology Information.7 A near infrared light-emitting diode developed by NASA has been used to heal and reduce pain for chemotherapy patients who subsequently suffer from mouth lesions.8 In the past fifty years, we’ve harnessed this invisible radiation to vastly improve our lives.










