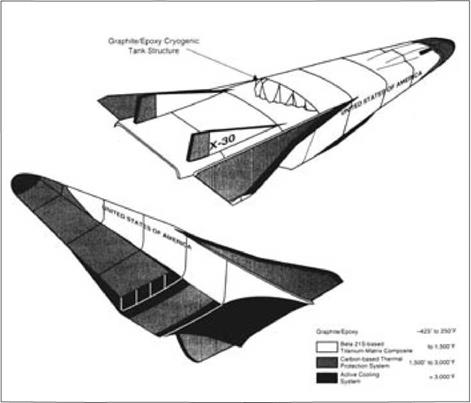
Materials
No aircraft has ever cruised at Mach 5, and an important reason involves structures and materials. “If I cruise in the atmosphere for two hours,” says Paul Czysz of McDonnell Douglas, “I have a thousand times the heat load into the vehicle that the shuttle gets on its quick transit of the atmosphere.” The thermal environment of
the X-30 was defined by aerodynamic heating and by the separate issue of flutter.48
A single concern dominated issues of structural design: The vehicle was to fly as low as possible in the atmosphere during ascent to orbit. Re-entry called for flight at higher altitudes, and the loads during ascent therefore were higher than those of re-entry. Ascent at lower altitude—200,000 feet, for instance, rather than 250,000—increased the drag on the X-30. But it also increased the thrust, giving a greater margin between thrust and drag that led to increased acceleration. Considerations of ascent, not re-entry, therefore shaped the selection of temperature-resistant materials.
Yet the aircraft could not fly too low, or it would face limits set by aerodynamic flutter. This resulted from forces on the vehicle that were not steady but oscillated, at frequencies of oscillation that changed as the vehicle accelerated and lost weight. The wings tended to vibrate at characteristic frequencies, as when bent upward and released to flex up and down. If the frequency of an aerodynamic oscillation matched that at which the wings were prone to flex, the aerodynamic forces could tear the wings off. Stiffness in materials, not strength, was what resisted flutter, and the vehicle was to fly a “flutter-limited trajectory,” staying high enough to avoid the problem.

 The mechanical properties of metals depend on their finegrained structure. An ingot of metal consists of a mass of interlaced grains or crystals, and small grains give higher strength. Quenching, plunging hot metal into water, yields small grains but often makes the metal brittle or hard to form. Alloying a metal, as by adding small quantities of
The mechanical properties of metals depend on their finegrained structure. An ingot of metal consists of a mass of interlaced grains or crystals, and small grains give higher strength. Quenching, plunging hot metal into water, yields small grains but often makes the metal brittle or hard to form. Alloying a metal, as by adding small quantities of
carbon to make steel, A. . . . .
Ascent trajectory or an airbreatner. (IN ASA)
is another traditional practice. However,
some additives refuse to dissolve or separate out from the parent metal as it cools.
To overcome such restrictions, techniques of powder metallurgy were in the forefront. These methods gave direct control of the microstructure of metals by forming
them from powder, with the grains of powder sintering or welding together by being pressed in a mold at high temperature. A manufacturer could control the grain size independently of any heat-treating process. Powder metallurgy also overcame restrictions on alloying by mixing in the desired additives as powdered ingredients.
Several techniques existed to produce the powders. Grinding a metal slab to sawdust was the simplest, yielding relatively coarse grains. “Splat-cooling” gave better control. It extruded molten metal onto the chilled rim of a rotating wheel, which cooled it instantly into a thin ribbon. This represented a quenching process that produced a fine-grained microstructure in the metal. The ribbon then was chemically treated with hydrogen, which made it brittle, so that it could be ground into a fine powder. Heating the powder then drove off the hydrogen.
The Plasma Rotating Electrode Process, developed by the firm of Nuclear Metals, showed particular promise. The parent metal was shaped into a cylinder that rotated at up to 30,000 revolutions per minute and served as an electrode. An electric arc melted the spinning metal, which threw off droplets within an atmosphere of cool inert helium. The droplets plummeted in temperature by thousands of degrees within milliseconds, and their microstructures were so fine as to approach an amorphous state. Their molecules did not form crystals, even tiny ones, but arranged themselves in formless patterns. This process, called “rapid solidification,” promised particular gains in high-temperature strength.
Standard titanium alloys, for instance, lost strength at temperatures above 700 to 900°E By using rapid solidification, McDonnell Douglas raised this limit to 1,100°F prior to 1986. Philip Parrish, the manager of powder metallurgy at DARPA, noted that his agency had spent some $30 million on rapid-solidification technology since 1975. In 1986 he described it as “an established technology. This technology now can stand along such traditional methods as ingot casting or drop forging.”49
Nevertheless 1,100°F was not enough, for it appeared that the X-30 needed a material that was rated at 1,700°F. This stemmed from the fact that for several years, NASP design and trajectory studies indicated that a flight vehicle indeed would face such temperatures on its fuselage. But after 1990 the development of new baseline configurations led to an appreciation that the pertinent areas of the vehicle would face temperatures no higher than 1,500°F. At that temperature, advanced titanium alloys could serve in “metal matrix composites,” with thin-gauge metals being reinforced with fibers.
The new composition came from the firm of Titanium Metals and was designated Beta-21S. That company developed it specifically for the X-30 and patented it in 1989- It consisted of titanium along with 15 percent molybdenum, 2.8 percent columbium, 3 percent aluminum, and 0.2 percent silicon. Resistance to oxidation proved to be its strong suit, with this alloy showing resistance that was two orders of magnitude greater than that of conventional aircraft titanium. Tests showed that it
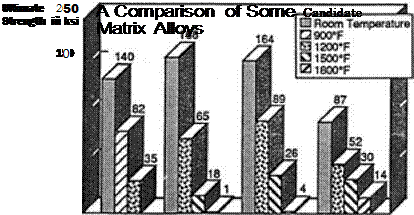 |
Comparison of some matrix alloys. (NASA)
also could be exposed repeatedly to leaks of gaseous hydrogen without being subject to embrittlement. Moreover, it lent itself readily to being rolled to foil-gauge thicknesses of 4 to 5 mil when metal matrix composites were fabricated.50
Such titanium-matrix composites were used in representative X-30 structures. The Non-Integral Fuselage Tank Article (NIFTA) represented a section of X-30 fuselage at one-fourth scale. It was oblong in shape, eight feet long and measuring four by seven feet in cross section, and it contained a splice. Its skin thickness was 0.040 inches, about the same as for the X-30. It held an insulated tank that could hold either liquid nitrogen or LH, in tests, which stood as a substantial engineering item in its own right.
The tank had a capacity of 940 gallons and was fabricated of graphite-epoxy composite. No liner protected the tankage on the inside, for graphite-epoxy was impervious to damage by LH. However, the exterior was insulated with two halfinch thicknesses of Q-felt, a quartz-fiber batting with density of only 3-5 pounds per cubic foot. A thin layer of Astroquartz high-temperature cloth covered the Q-felt. This insulation filled space between the tank wall and the surrounding wall of the main structure, with both this space and the Q-felt being purged with helium.51
The test sequence for NIFTA duplicated the most severe temperatures and stresses of an ascent to orbit. These stresses began on the ground, with the vehicle being heavy with fuel and subject to a substantial bending load. There was also a
large shear load, with portions of the vehicle being pulled transversely in opposite directions. This happened because the landing gear pushed upward to support the entire weight of the craft, while the weight of the hydrogen tank pushed downward only a few feet away. Other major bending and shear loads arose during subsonic climbout, with the X-30 executing a pullup maneuver.
Significant stresses arose near Mach 6 and resulted from temperature differences across the thickness of the stiffened skin. Its outer temperature was to be 800°F, but the tops of the stiffeners, a few inches away, were to be 350°F. These stiffeners were spot-welded to the skin panels, which raised the issue of whether the welds would hold amid the different thermal expansions. Then between Mach 10 and 16, the vehicle was to reach peak temperatures of 1,300°F. The temperature differences between the top and bottom of the vehicle also would be at their maximum.
The tests combined both thermal and mechanical loads and were conducted within a vacuum chamber at Wyle Laboratories during 1991- Banks of quartz lamps applied up to 1.5 megawatts of heat, while jacks imposed bending or shear forces that reached 100 percent of the design limits. Most tests placed nonflammable liquid nitrogen in the tank for safety, but the last of them indeed used LHr With this supercold fuel at -423°F, the lamps raised the exterior temperature of NIFTA to the full 1,300°F, while the jacks applied the full bending load. A 1993 paper noted “100% successful completion of these tests,” including the one with LH2 that had been particularly demanding.52
NIFTA, again, was at one-fourth scale. In a project that ran from 1991 through the summer of 1994, McDonnell Douglas engineers designed and fabricated the substantially larger Full Scale Assembly. Described as “the largest and most representative NASP fuselage structure built,” it took shape as a component measuring 10 by 12 feet. It simulated a section of the upper mid-fuselage, just aft of the crew compartment.
A1994 review declared that it “was developed to demonstrate manufacturing and assembly of a full scale fuselage panel incorporating all the essential structural details of a flight vehicle fuselage assembly.” Crafted in flightweight, it used individual panels of titanium-matrix composite that were as large as four by eight feet. These were stiffened with longitudinal members of the same material and were joined to circumferential frames and fittings of Ti-1100, a titanium alloy that used no fiber reinforcement. The complete assembly posed manufacturing challenges because the panels were of minimum thickness, having thinner gauges than had been used previously. The finished article was completed just as NASP was reaching its end, but it showed that the thin panels did not introduce significant problems.53
The firm of Textron manufactured the fibers, designated SCS-6 and -9, that reinforced the composites. As a final touch, in 1992 this company opened the worlds first manufacturing plant dedicated to the production of titanium-matrix
composites. “We could get the cost down below a thousand dollars a pound if we had enough volume,” Bill Grant, a company manager, told Aerospace America. His colleague Jim Henshaw added, “We think SCS/titanium composites are fully developed for structural applications.”54
Such materials served to 1,500°F, but on the X-30 substantial areas were to withstand temperatures approaching 3,000°F, which is hotter than molten iron. If a steelworker were to plunge a hand into a ladle of this metal, the hand would explode from the sudden boiling of water in its tissues. In such areas, carbon-carbon was necessary. It had not been available for use in Dyna-Soar, but the Pentagon spent $200 million to fund its development between 1970 and 1985.55
Much of this supported the space shuttle, on which carbon-carbon protected such hot areas as the nose cap and wing leading edges. For the X-30, these areas expanded to cover the entire nose and much of the vehicle undersurface, along with the rudders and both the top and bottom surfaces of the wings. The X-30 was to execute 150 test flights, exposing its heat shield to prolonged thermal soaks while still in the atmosphere. This raised the problem of protection against oxidation.56
|
Selection of NASP materials based on temperature. (General Accounting Office) |
Standard approaches called for mixing oxidation inhibitors into the carbon matrix and covering the surface with a coating of silicon carbide. However, there was a mismatch between the thermal expansions of the coating and the carbon – carbon substrate, which led to cracks. An interlayer of glass-forming sealant, placed between them, produced an impervious barrier that softened at high temperatures to fill the cracks. But these glasses did not flow readily at temperatures below 1,500°F. This meant that air could penetrate the coating and reach the carbon through open cracks to cause loss by oxidation.57
The goal was to protect carbon-carbon against oxidation for all 150 of those test flights, or 250 hours. These missions included 75 to orbit and 75 in hypersonic cruise. The work proceeded initially by evaluating several dozen test samples that were provided by commercial vendors. Most of these materials proved to resist oxidation for only 10 to 20 hours, but one specimen from the firm of Hitco reached 70 hours. Its surface had been grooved to promote adherence of the coating, and it gave hope that long operational life might be achieved.58
Complementing the study of vendors’ samples, researchers ordered new types of carbon-carbon and conducted additional tests. The most durable came from the firm of Rohr, with a coating by Science Applications International. It easily withstood 2,000°F for 200 hours and was still going strong at 2,500 °F when the tests stopped after 150 hours. This excellent performance stemmed from its use of large quantities of oxidation inhibitors, which promoted long life, and of multiple glass layers in the coating.
But even the best of these carbon-carbons showed far poorer performance when tested in arcjets at 2,500°F. The high-speed airflows forced oxygen into cracks and pores within the material, while promoting evaporation of the glass sealants. Powerful roars within the arcjets imposed acoustic loads that contributed to cracking, with other cracks arising from thermal shock as test specimens were suddenly plunged into a hot flow stream. The best results indicated lifetimes of less than two hours.
Fortunately, actual X-30 missions were to impose 2,500°F temperatures for only a few minutes during each launch and reentry. Even a single hour of lifetime therefore could permit panels of carbon-carbon to serve for a number of flights. A 1992 review concluded that “maximum service temperatures should be limited to 2,800°F; above this temperature the silicon-based coating systems afford little practical durability,” due to active oxidation. In addition, “periodic replacement of parts may be inevitable.”59
New work on carbon-carbon, reported in 1993, gave greater encouragement as it raised the prospect of longer lifetimes. The effort evaluated small samples rather than fabricated panels and again used the arcjet installations of NASA-Johnson and Ames. Once again there was an orders-of-magnitude difference in the observed lifetimes of the carbon-carbon, but now the measured lifetimes extended into the hundreds of minutes. A formulation from the firm of Carbon-Carbon Advanced
Technologies gave the best results, suggesting 25 reuses for orbital missions of the X-30 and 50 reuses for the less-demanding missions of hypersonic cruise.60
There also was interest in using carbon-carbon for primary structure. Here the property that counted was not its heat resistance but its light weight. In an important experiment, the firm of LTV fabricated half of an entire wing box of this material. An airplanes wing box is a major element of aircraft structure that joins the wings and provides a solid base for attachment of the fuselage fore and aft. Indeed, one could compare it with the keel of a ship. It extends to left and right of the aircraft centerline, and LTV s box constituted the portion to the left of this line. Built at full scale, it represented a hot-structure wing proposed by General Dynamics. It measured five by eight feet with a maximum thickness of 16 inches. Three spars ran along its length; five ribs were mounted transversely, and the complete assembly weighed 802 pounds.
The test plan called for it to be pulled upward at the tip to reproduce the bending loads of a wing in flight. Torsion or twisting was to be applied by pulling more strongly on the front or rear spar. The maximum load corresponded to having the X – 30 execute a pullup maneuver at Mach 2.2, with the wing box at room temperature. With the ascent continuing and the vehicle undergoing aerodynamic heating, the next key event brought the maximum difference in the temperatures of the top and bottom of the wing box, with the former being 994°F and the latter at 1,671°F. At that moment the load on the wing box corresponded to 34 percent of the Mach 2.2 maximum. Farther along, the wing box was to reach its peak temperature, 1,925°F, on the lower surface. These three points were to be reproduced through mechanical forces applied at the ends of the spars and through the use of graphite heaters.
But several key parts delaminated during their fabrication, seriously compromising the ability of the wing box to bear its specified load. Plans to impose the peak or Mach 2.2 load were abandoned, with the maximum planned load being reduced to the 34 percent associated with the maximum temperature difference. For the same reason, the application of torsion was deleted from the test program. Amid these reductions in the scope of the structural tests, two exercises went forward during December 1991. The first took place at room temperature and successfully reached the mark of 34 percent, without causing further damage to the wing box.
The second test, a week later, reproduced the condition of peak temperature difference while briefly applying the calculated load of 34 percent. The plan then called for further heating to the peak temperature of 1,925°F. As the wing box approached this value, a problem arose due to the use of metal fasteners in its assembly. Some were made from coated columbium and were rated for 2,300°F, but most were of a nickel alloy that had a permissible temperature of 2,000°F. However, an instrumented nickel-alloy fastener overheated and reached 2,l47°F- The wing box showed a maximum temperature of 1,917°F at that moment, and the test was terminated because the strength of the fasteners now was in question. This test nevertheless
counted as a success because it had come within 8°F of the specified temperature.61
Both tests thus were marked as having achieved their goals, but their merits were largely in the mind of the beholder. The entire project would have been far more impressive if it had avoided delamination, successfully achieved the Mach 2.2 peak load, incorporated torsion, and subjected the wing box to repeated cycles of bending, torsion, and heating. This effort stood as a bold leap toward a future in which carbon-carbon might take its place as a mainstream material, suitable for a hot primary structure, but it was clear that this future would not arrive during the NASP program.
Then there was beryllium. It had only two-thirds the density of aluminum and possessed good strength, but its temperature range was limited. The conventional metal had a limit of some 850°F, but an alloy from Lockheed called Lockalloy, which contained 38 percent aluminum, was rated only for 600°F. It had never become a mainstream engineering material like titanium, but for NASP it offered the advantage of high thermal conductivity. Work with titanium had greatly increased its temperatures of use, and there was hope of achieving similar results with beryllium.
Initial efforts used rapid-solidification techniques and sought temperature limits as high as 1,500°F. These attempts bore no fruit, and from 1988 onward the temperature goal fell lower and lower. In May 1990 a program review shifted the emphasis away from high-temperature formulations toward the development of beryllium as a material suitable for use at cryogenic temperatures. Standard forms of this metal became unacceptably brittle when only slightly colder than ~100°F, but cryo-beryl – lium proved to be out of reach as well. By 1992 investigators were working with ductile alloys of beryllium and were sacrificing all prospect of use at temperatures beyond a few hundred degrees but were winning only modest improvements in low – temperature capability. Terence Ronald, the NASP materials director, wrote in 1995 of rapid-solidification versions with temperature limits as low as 500°F, which was not what the X-30 needed to reach orbit.62
In sum, the NASP materials effort scored a major advance with Beta-21S, but the genuinely radical possibilities failed to emerge. These included carbon-carbon as primary structure, along with alloys of beryllium that were rated for temperatures well above 1,000°F. The latter, if available, might have led to a primary structure with the strength and temperature resistance of Beta-2 IS but with less than half the weight. Indeed, such weight savings would have ramified through the entire design, leading to a configuration that would have been smaller and lighter overall.
Overall, work with materials fell well short of its goals. In dealing with structures and materials, the contractors and the National Program Office established 19 program milestones that were to be accomplished by September 1993- A General Accounting Office program review, issued in December 1992, noted that only six of them would indeed be completed.63 This slow progress encouraged conservatism in drawing up the bill of materials, but this conservatism carried a penalty.
When the scramjets faltered in their calculated performance and the X-30 gained weight while falling short of orbit, designers lacked recourse to new and very light materials—structural carbon-carbon, high-temperature beryllium—that might have saved the situation. With this, NASP spiraled to its end. It also left its supporters with renewed appreciation for rockets as launch vehicles, which had been flying to orbit for decades.