Melting Your Troubles Away
As quickly as the hazards of aircraft icing became known in the early days of aviation, inventive spirits applied themselves to coming up with ways to remove the hazard and allow the airplane to keep flying. These ideas at first took the form of understanding where and when icing occurs and then simply not flying through such conditions, then ways to prevent ice from forming in the first place—proactive anti-icing— were considered, and at the same time options for removing ice once it
 |
|
had formed—reactive de-icing—were suggested and tested in the field, in the air, and in the wind tunnel. Of all the options available, the three major ones are the pneumatic boot, spraying chemicals onto the aircraft, and channeling hot bleed air.[1224]
The oldest of the de-icing methods in use is the pneumatic boot system, invented in 1923 by the B. F. Goodrich Corporation in Akron, OH. The general idea behind the boot has not changed nearly a century later: a thick rubber membrane is attached to the leading edge of a wing airfoil. Small holes in the wing behind the boot allow compressed air to blow through, ever so slightly expanding the boot’s volume like a balloon. Any time that ice builds up on the wing, the system is activated, and when the boot expands, it essentially breaks the ice into pieces, which are quickly blown away by the relative wind of the moving aircraft. Again, although the general design of the boot system has not
changed, there have been improvements in materials science and sensor technology, as well as changes in the shape of wings used in various sizes and types of aircraft. In this manner, NASA researchers have been very active in coming up with new and inventive ways to enhance the original boot concept and operation.[1225]
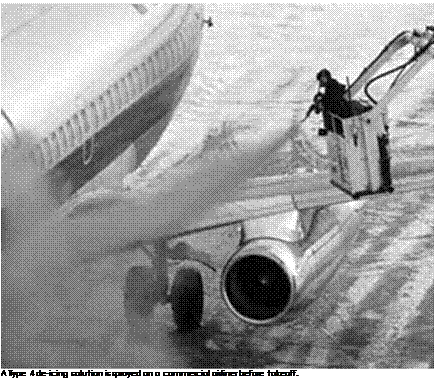
![]() One way to ensure there is no ice on an aircraft is to remove it before the flight gets off the ground. The most common method for doing this is to spray some type of de-icing fluid onto the aircraft surface as close to takeoff as possible. The idea was first proposed by Joseph Halbert and used by the United Kingdom Royal Air Force in 1937 on the large flying boats then operated by Imperial Airways.[1226] Today, the chemicals used in these fluids usually use a propylene glycol or ethylene glycol base and may include other ingredients that might thicken the fluid, help inhibit corrosion on the aircraft, or add a color to the mixture for easier identification. Often water is added to the mixture, which although counterintuitive makes the liquid more effective. Of the two glycols, propylene is more environmentally friendly.[1227]
One way to ensure there is no ice on an aircraft is to remove it before the flight gets off the ground. The most common method for doing this is to spray some type of de-icing fluid onto the aircraft surface as close to takeoff as possible. The idea was first proposed by Joseph Halbert and used by the United Kingdom Royal Air Force in 1937 on the large flying boats then operated by Imperial Airways.[1226] Today, the chemicals used in these fluids usually use a propylene glycol or ethylene glycol base and may include other ingredients that might thicken the fluid, help inhibit corrosion on the aircraft, or add a color to the mixture for easier identification. Often water is added to the mixture, which although counterintuitive makes the liquid more effective. Of the two glycols, propylene is more environmentally friendly.[1227]
The industry standard for this fluid is set by the aeronautics division of the Society of Automotive Engineers, which has published standards for four types of de-icing fluids, each with slightly different properties and intentions for use. Type I has a low viscosity and is usually heated and sprayed on aircraft at high pressure to remove any snow, ice, or frost. Due to its viscosity, it runs off the aircraft very quickly and provides little to no protection as an anti-icing agent as the aircraft is exposed to snowy or icy conditions before takeoff. Its color is usually orange.[1228] Type II fluid has a thickening agent to prevent it from running very quickly off the aircraft, leaving a film behind that acts as an anti-icing agent until the aircraft reaches a speed of 100 knots, when the fluid breaks down from aerodynamic stress. The fluid is usually light yellow. Type III fluid’s properties fall in between Type I and II, and it is intended for smaller,
 |
|
slower aircraft. It is popular in the regional and business aviation markets and is usually dyed light yellow. Type IV fluids are only applied after a Type I fluid is sprayed on to remove all snow, ice, and frost. The Type IV fluid is designed to leave a film on the aircraft that will remain for 30 to 80 minutes, serving as a strong anti-icing agent. It is usually green.[1229]
NASA researchers have worked with these fluids for many years and found uses in other programs, including the International Space Station. And during the late 1990s, a team of engineers from the Ames Research Center (ARC) at Moffett Field, CA, came up with an anti-icing fluid that was nontoxic—so much so that it was deemed "food grade” because its ingredients were approved by the U. S. Government for use in food— namely ice cream—and promised to last longer as an anti-icing agent for aircraft, as well as work as an effective de-icing agent. Although it
has not found wide use in the aviation industry, NASA did issue a license to a commercial firm who now sells the product to consumers as "Ice Free,” a spray for automobile windshields that can provide protection from snow or ice forming on a windshield in temperatures down to 20 degrees Fahrenheit (-7 degrees Celsius).[1230]
![]() The third common technique for dealing with ice accretion is the hot bleed air method. In this scheme, hot air is channeled away from the aircraft engines and fed into tubes that run throughout the aircraft near the areas where ice is most likely to form and do the most damage. The hot air warms the aircraft skin, melting away any ice that is there and discouraging any ice from forming. The hot gas can also be used as the source of pressurized air that inflates a rubber boot, if one is present. While the idea of using hot bleed air became most practical with the introduction of jet engines, the basic concept itself dates back to the 1930s, when NACA engineers proposed the idea and tested it in an open-air-cockpit, bi-wing airplane. The in-flight experiments showed that "a vapor-heating system which extracts heat from the exhaust and distributes it to the wings is an entirely practical and efficient method for preventing ice formation.”[1231]
The third common technique for dealing with ice accretion is the hot bleed air method. In this scheme, hot air is channeled away from the aircraft engines and fed into tubes that run throughout the aircraft near the areas where ice is most likely to form and do the most damage. The hot air warms the aircraft skin, melting away any ice that is there and discouraging any ice from forming. The hot gas can also be used as the source of pressurized air that inflates a rubber boot, if one is present. While the idea of using hot bleed air became most practical with the introduction of jet engines, the basic concept itself dates back to the 1930s, when NACA engineers proposed the idea and tested it in an open-air-cockpit, bi-wing airplane. The in-flight experiments showed that "a vapor-heating system which extracts heat from the exhaust and distributes it to the wings is an entirely practical and efficient method for preventing ice formation.”[1231]
As for melting ice that can accrete on or in other parts of an aircraft, such as windshields, protruding Pitot tubes, antennas, and carburetors on piston engines, electrically powered heaters of one kind or another are employed. The problem of carburetor ice is especially important and the one form of icing most prevalent and dangerous for thousands of General Aviation pilots. NASA has studied carburetor ice for engines and aircraft of various configurations through the years[1232] and in 1975 surveyed the accident database and found that between 65 and 90 accidents each year involve carburetor icing as the probable cause. And when there are known carburetor icing conditions, between 50 and 70 percent of engine failure accidents are due to carburetor icing. Researchers found the problem to be particularly acute for pilots
with less than 1,000 hours of total flying time and overall exposed about 144 persons to death or injury each year.[1233]










