Hydrogen Research Leads to Rockets and Fuel Cells
While most of NASA’s aircraft fuel-efficiency research grew out of the reality jolt of the 1970s oil crisis and environmental concerns, there is at least one notable exception. Researchers first began to investigate liquid hydrogen as an alternative to hydrocarbon-based fuel in the mid – 1950s because they suspected major performance efficiencies could be gained.[1468] The Lewis Flight Propulsion Laboratory issued a seminal report in April 1955—although it was not declassified until September 1962—suggesting that liquid hydrogen might have a positive impact on the performance of high-altitude military aircraft (subsonic and supersonic bombers, fighters, and reconnaissance aircraft flying at 75,000 to 85,000 feet).[1469] While the report raised the aviation community’s awareness of the potential for hydrogen as a fuel source, it did not lead to widespread use in aircraft because of technical problems with using
 |
|
hydrogen inside an aircraft. Again, this early interest in hydrogen did not reflect environmental or conservation concerns, but rather, a desire to achieve much higher flight vehicle performance.
In the report, two NACA researchers—Abe Silverstein and Eldon Hall—argued the case for using liquid hydrogen, noting it has a special advantage as an aviation fuel: a high heating value. This means that it takes less hydrogen fuel than hydrocarbon fuel to achieve the same thrust. That advantage could prove particularly important at high altitudes, the researchers noted, where maximizing fuel efficiency is critical to make up for other penalties associated with flying high.
Indeed, one of the downsides of a high-altitude flight regime—in which atmospheric pressure is low—is that it generally requires heavy, high-pressure-ratio engines to ensure combustion is sustained and thrust levels are adequate. The NACA report speculated that it might be possible to use lighter engines—albeit less-efficient ones, with lower pressure ratios—if liquid hydrogen were used for fuel. Liquid hydrogen requires less combustion volume than hydrocarbons do, making shorter and lighter engines feasible. And, with its high heating value, liquid hydrogen fuel generates more thrust per pound than hydrocarbons do, even if it’s being used in a light engine running at a lower pressure ratio. The report posited that if every pound of weight saved by using a lighter
 |
|
engine could be replaced by a pound of liquid hydrogen fuel, an aircraft could be over twice as effective in extending its range at high altitudes.
After Silverstein and Hall issued their report, the NACA conducted experiments showing that hydrogen had a high combustion efficiency in a turbojet combustor even in low-pressure conditions. In 1956, NACA researchers at Lewis made "three completely successful flights” using liquid hydrogen in one engine of a modified Martin B-57B jet bomber, thereby effectively demonstrating that liquid hydrogen could be handled and jettisoned safely and was feasible for use in aircraft.[1470] Meanwhile, from 1956 to 1958, the U. S. Air Force began work on a secret project, known as Suntan, to develop a high-altitude, hydrogen-fueled aircraft with performance superior to the secret U-2 spy plane of the Counter Intelligence Agency (CIA).
The use of liquid hydrogen in aircraft would have marked a major breakthrough in terms of high altitude flight because engine weight is
"the single most important variable determining the height at which an airplane can fly.”[1471] Liquid hydrogen offered the potential to fly at high altitudes at an extended range. Despite its potential, however, neither the NACA nor the Air Force was able to convince enough stakeholders in Government and industry that liquid hydrogen was a viable candidate for aviation fuel.
![]() Hydrogen’s excellent combustion qualities raised questions about whether it could be safely transported or carried inside aircraft. To be sure, NACA flight tests demonstrated that safe handling of hydrogen fuel on the ground and in the air was possible. Also, the Air Force conducted tests in which liquid hydrogen tanks under pressure were ruptured, and it found that in many cases the hydrogen quickly escaped without ignition. Yet concerns about safety persisted, and, in a tight budget climate, hydrogen-fuelled aircraft lost out to other priorities. After receiving a full briefing on Suntan, Gen. Curtis E. Lemay, the former head of Strategic Air Command who had moved up to Vice Chief of Staff in July 1957, raised concerns about safety. "What,” he said, "put my pilots up there with a bomb?”[1472]
Hydrogen’s excellent combustion qualities raised questions about whether it could be safely transported or carried inside aircraft. To be sure, NACA flight tests demonstrated that safe handling of hydrogen fuel on the ground and in the air was possible. Also, the Air Force conducted tests in which liquid hydrogen tanks under pressure were ruptured, and it found that in many cases the hydrogen quickly escaped without ignition. Yet concerns about safety persisted, and, in a tight budget climate, hydrogen-fuelled aircraft lost out to other priorities. After receiving a full briefing on Suntan, Gen. Curtis E. Lemay, the former head of Strategic Air Command who had moved up to Vice Chief of Staff in July 1957, raised concerns about safety. "What,” he said, "put my pilots up there with a bomb?”[1472]
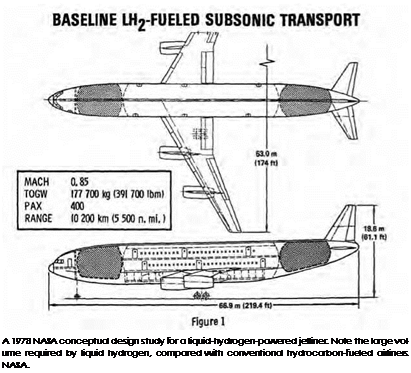
Others questioned whether using liquid hydrogen would truly yield big gains in aircraft range at high altitudes. Hydrogen has a high volume—10 times that of hydrocarbons—which means that the aircraft fuselage has to be bigger and weigh more to accommodate the fuel. Silverstein and Hall argued that there would be more room for hydrogen fuel tanks in high-altitude aircraft, which would need larger wings and therefore a bigger fuselage to provide lift in the thin air of the upper atmosphere (a bigger fuselage would mean more room for hydrogen fuel tanks). But while it might have been possible to extend the range of aircraft because of the increased efficiency of liquid hydrogen, others questioned whether hydrogen-fuelled aircraft would still be fairly limited by the tremendous amount of fuel storage capacity that hydrogen requires. Kelly Johnson, the Lockheed Martin engineer who designed the U-2 and the hydrogen-fueled CL-400 for the Air Force’s Suntan project, said he could see a range growth of only 3 percent from adding more hydrogen fuel storage capacity to his CL-400 design. "We have crammed the max
imum amount of hydrogen in the fuselage that it can hold. You do not carry hydrogen in the flat surfaces of the wing,” he said.[1473]
![]() While liquid hydrogen is highly energetic, it has far less energy density than hydrocarbon fuels. Thus, to get an equivalent amount of energy from hydrogen requires a much greater volume. Accordingly, a hydrogen airplane would have extremely large fuel tanks, which, having to be supercold as well, pose significant technical challenges to aircraft designers. Researchers have not yet found a way to overcome the challenges associated with hydrogen’s large volume, which forces aircraft design compromises and requires complex ground transportation, storage distribution, and vent capture system. Moreover, hydrogen is not a viable source of energy in itself; producing it requires the use of other sources of energy—such as electric power produced by nuclear fusion as well as a large source of clean water. However, in one respect, hydrogen could "pay back” this "debt,” for it could be used to enrich the production process of synthetic fuel, achieving similar production efficiencies while reducing the amount of water and coal traditionally required for enrichment.[1474]
While liquid hydrogen is highly energetic, it has far less energy density than hydrocarbon fuels. Thus, to get an equivalent amount of energy from hydrogen requires a much greater volume. Accordingly, a hydrogen airplane would have extremely large fuel tanks, which, having to be supercold as well, pose significant technical challenges to aircraft designers. Researchers have not yet found a way to overcome the challenges associated with hydrogen’s large volume, which forces aircraft design compromises and requires complex ground transportation, storage distribution, and vent capture system. Moreover, hydrogen is not a viable source of energy in itself; producing it requires the use of other sources of energy—such as electric power produced by nuclear fusion as well as a large source of clean water. However, in one respect, hydrogen could "pay back” this "debt,” for it could be used to enrich the production process of synthetic fuel, achieving similar production efficiencies while reducing the amount of water and coal traditionally required for enrichment.[1474]
Despite these technical challenges, NASA’s research on the use of hydrogen to power aircraft did lead to some important findings: namely, that hydrogen is a potentially promising turbojet fuel in a high-altitude, low-speed flight regime. These conditions favor a fuel that can operate efficiently in low-pressure conditions. High altitudes also favor a large – volume aircraft, helping to offset the disadvantage of hydrogen’s low density. Given these attractive characteristics, the prospect of using hydrogen as an aircraft propellant has continued to resurface in the past decade, especially when the cost of hydrocarbon-based fuel rises. For example, NASA’s Zero CO2 research project sought to eliminate carbon dioxide and lower NOx emissions by converting propulsion systems to hydrogen fuel.[1475] One new propulsion technology that NASA engineers considered as part of Zero CO2 was the use of fuel cells, which are discussed below. A NASA Glenn Web page updated as recently as 2008 says that the Combustion Branch of NASA’s Propulsion Division is still studying
hydrogen combustion to demonstrate that hydrogen can be used as an aviation fuel to minimize emissions.[1476]
![]() The NACA’s early research on hydrogen-fuelled aircraft also created an awareness of hydrogen as a potential fuel source that did not exist before Silverstein and Hall embarked on their study. This awareness helped lead to important breakthroughs in rocketry and fuel cell research. In particular, research on the use of hydrogen in air-breathing aircraft laid the groundwork for the successful development of hydrogen-fueled rockets in the mid-1950s. In fact, Silverstein and Hall’s research helped to inform NASA’s decision in 1959 to use liquid hydrogen as a propellant in the upper stage of the Saturn launch vehicle. That decision was one of the keys to the success of the Apollo Moon landing missions of the 1960s and 1970s.[1477]
The NACA’s early research on hydrogen-fuelled aircraft also created an awareness of hydrogen as a potential fuel source that did not exist before Silverstein and Hall embarked on their study. This awareness helped lead to important breakthroughs in rocketry and fuel cell research. In particular, research on the use of hydrogen in air-breathing aircraft laid the groundwork for the successful development of hydrogen-fueled rockets in the mid-1950s. In fact, Silverstein and Hall’s research helped to inform NASA’s decision in 1959 to use liquid hydrogen as a propellant in the upper stage of the Saturn launch vehicle. That decision was one of the keys to the success of the Apollo Moon landing missions of the 1960s and 1970s.[1477]
The NACA’s early efforts to draw attention to hydrogen as a power source also led to the development of fuel cells for the Apollo and Gemini capsules. Apollo employed the world’s first fuel cells, which used hydrogen and oxygen to generate onboard power for Apollo command and service modules. Fuel cells are essentially plastic membranes treated with a special catalyst; hydrogen seeps into the membrane and meets up with the oxygen inside to generate electricity and water. The fuel cells used on the Apollo proved so successful that they were once again employed on the Space Shuttle orbiter.










