Hot Structures: Dyna-Soar
Reentry of ICBM nose cones and of satellites takes place at nearly the same velocity. Reentry of spacecraft takes place at a standard velocity of Mach 25, but there are large differences in the technical means that have been studied for the thermal protection. During the 1960s, it was commonly expected that such craft would be built as hot structures. In fact, however, the thermal protection adopted for the Shuttle was the well-known "tiles,” a type of reusable insulation.
The Dyna-Soar program, early in the ’60s, was first to face this issue. Dyna-Soar used a radiatively cooled hot structure, with the primary or load-bearing structure being of Rene 41. Trusses formed the primary structure of the wings and fuselage, with many of their beams meeting at joints that were pinned rather than welded. Thermal gradients,
 PILOTS HATCH
PILOTS HATCH
![]() ROLL REACTION CONTROL
ROLL REACTION CONTROL
![]() PITCH REACTION CONTROL ANTENNAS
PITCH REACTION CONTROL ANTENNAS
YAW REACTION CONTROL 35.34 FT.
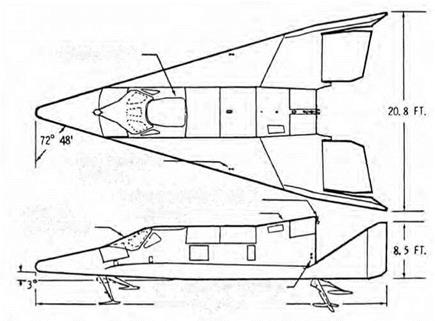
Schematic drawing of the Boeing X-20A Dyna-Soar. USAF.
imposing differential expansion on separate beams, caused these members to rotate at the pins. This accommodated the gradients without imposing thermal stresses. Rene 41 was selected as a commercially available superalloy that had the best available combination of oxidation resistance and high-temperature strength. Its yield strength,
130,0 pounds per square inch (psi) at room temperature, fell off only slightly at 1,200 °F and retained useful values at 1,800 °F. It could be processed as sheet, strip, wire, tubes, and forgings. Used as primary structure of Dyna-Soar, it supported a design specification that stated that the craft was to withstand at least four reentries under the most severe conditions permitted.
As an alloy, Rene 41 had a standard composition of 19 percent chromium, 11 percent cobalt, 10 percent molybdenum, 3 percent titanium, and 1.5 percent aluminum, along with 0.09 percent carbon and 0.006 percent boron, with the balance being nickel. It gained strength through age hardening, with the titanium and aluminum precipitating within the nickel as an intermetallic compound. Age-hardening weldments initially showed susceptibility to cracking, which occurred in parts that had been strained through welding or cold working. A new heat-treatment process
permitted full aging without cracking, with the fabricated assemblies showing no significant tendency to develop cracks.[1036]
As a structural material, the relatively mature state of Rene 41 reflected the fact that it had already seen use in jet engines. It nevertheless lacked the temperature resistance necessary for use in the metallic shingles or panels that were to form the outer skin of the vehicle, which were to reradiate the heat while withstanding temperatures as high as
3,0 °F. Here there was far less existing art, and investigators at Boeing had to find their way through a somewhat roundabout path. Four refractory or temperature-resistant metals initially stood out: tantalum, tungsten, molybdenum, and columbium. Tantalum was too heavy. Tungsten was not available commercially as sheet. Columbium also appeared to be ruled out, for it required an antioxidation coating, but vendors were unable to coat it without rendering it brittle. Molybdenum alloys also faced embrittlement because of recrystallization produced by a prolonged soak at high temperature in the course of coating formation. A promising alloy, Mo-0.5Ti, overcame this difficulty through addition of
0. 07 percent zirconium. The alloy that resulted, Mo-0.5Ti-0.07Zr, was called TZM molybdenum. For a time it appeared as a highly promising candidate for all the other panels.[1037]
Wing design also promoted its use, for the craft mounted a delta wing with leading-edge sweep of 73 degrees. Though built for hypersonic entry from orbit, it resembled the supersonic delta wings of contemporary aircraft such as the B-58 bomber. But this wing was designed using H. Julian Allen’s blunt-body principle, with the leading edge being thickly rounded (that is, blunted) to reduce the rate of heating. The wing sweep then reduced equilibrium temperatures along the leading edge to levels compatible with the use of TZM.[1038]
Boeing’s metallurgists nevertheless held an ongoing interest in columbium, because in uncoated form it showed superior ease of fabrication and lack of brittleness. A new Boeing-developed coating method eliminated embrittlement, putting columbium back in the running. A survey of its alloys showed that they all lacked the hot strength of TZM. Columbium nevertheless retained its attractiveness because it promised less weight. Based on coatability, oxidation resistance, and thermal emis – sivity, the preferred alloy was Cb-10Ti-5Zr, called D-36. It replaced TZM in many areas of the vehicle but proved to lack strength against creep at the highest temperatures. Moreover, coated TZM gave more of a margin against oxidation than coated D-36 did, again at the most extreme temperatures. D-36 indeed was chosen to cover most of the vehicle, including the flat underside of the wing. But TZM retained its advantage for such hot areas as the wing leading edges.[1039]
The vehicle had some 140 running feet of leading edges and 140 square feet of associated area. This included leading edges of the vertical fins and elevons as well as of the wings. In general, D-36 served when temperatures during reentry did not exceed 2,700 °F, while TZM was used for temperatures between 2,700 and 3,000 °F. In accordance with the Stefan-Boltzmann law, all surfaces radiated heat at a rate proportional to the fourth power of the temperature. Hence for equal emissivities, a surface at 3,000 °F radiated 44 percent more heat than one at 2,700 °F.[1040]
Panels of both TZM and D-36 demanded antioxidation coatings. These coatings were formed directly on the surfaces as metallic silicides (silicon compounds), using a two-step process that employed iodine as a chemical intermediary. Boeing introduced a fluidized-bed method for application of the coatings that cut the time for preparation while enhancing uniformity and reliability. In addition, a thin layer of silicon carbide, applied to the surface, gave the vehicle its distinctive black color. It enhanced the emissivity, lowering temperatures by as much as 200 °F.
It was necessary to show that complete panels could withstand aerodynamic flutter. A report of the Aerospace Vehicles Panel of the Air Force Scientific Advisory Board came out in April 1962 and singled out the problem of flutter, citing it as one that called for critical attention. The test program used two NASA wind tunnels: the 4-foot by 4-foot Unitary facility at Langley that covered a range of Mach 1.6 to 2.8 and the 11-foot by 11-foot Unitary installation at Ames for Mach 1.2 to
1. 4. Heaters warmed test samples to 840 °F as investigators started with steel panels and progressed to versions fabricated from Rene nickel alloy.
"Flutter testing in wind tunnels is inherently dangerous,” a Boeing review declared. "To carry the test to the actual flutter point is to risk destruction of the test specimen. Under such circumstances, the safety of the wind tunnel itself is jeopardized.” Panels under test were as large as 24 by 45 inches; flutter could have brought failure through fatigue, with parts of a specimen being blown through the tunnel at supersonic speed. Thus, the work started at dynamic pressures of 400 and 500 pounds per square foot (psf) and advanced over a year and a half to exceed the design requirement of close to 1,400 psf. Tests were concluded in 1962.[1041]
Between the outer panels and the inner primary structure, a corrugated skin of Rene 41 served as the substructure. On the upper wing surface and upper fuselage, where the temperatures were no higher than 2,000 °F, the thermal-protection panels were also of Rene 41 rather than of a refractory. Measuring 12 by 45 inches, these panels were spot-welded directly to the corrugations of the substructure. For the wing undersurface and for other areas that were hotter than 2,000 °F, designers specified an insulated structure. Standoff clips, each with four legs, were riveted to the underlying corrugations and supported the refractory panels, which also were 12 by 45 inches in size.
The space between the panels and the substructure was to be filled with insulation. A survey of candidate materials showed that most of them exhibited a strong tendency to shrink at high temperatures. This was undesirable; it increased the rate of heat transfer and could create uninsulated gaps at seams and corners. Q-felt, a silica fiber from Johns Manville, also showed shrinkage. However, nearly all of it occurred at
2,0 °F and below; above 2,000 °F, further shrinkage was negligible. This meant that Q-felt could be "pre-shrunk” through exposure to temperatures above 2,000 °F for several hours. The insulation that resulted had density no greater than 6.2 pounds per cubic foot, one-tenth that of water. In addition, it withstood temperatures as high as 3,000 °F.[1042]
TZM outer panels, insulated with Q-felt, proved suitable for wing leading edges. These were designed to withstand equilibrium temperatures of 2,825 °F and short-duration over-temperatures of 2,900 °F. But the nose cap faced temperatures of 3,680 °F along with a peak heat flux of 143 BTU/ft2/sec. This cap had a radius of curvature of 7.5 inches, making it far less blunt than the contemporary Project Mercury heat shield that had a radius of 120 inches.[1043] Its heating was correspondingly more severe. Reliable thermal protection of the nose was essential, so the program conducted two independent development efforts that used separate technical approaches. The firm of Chance Vought pursued the main line of activity, while Boeing also devised its own nose cap design.
The work at Vought began with a survey of materials that paralleled Boeing’s review of refractory metals for the thermal-protection panels. Molybdenum and columbium had no strength to speak of at the pertinent temperatures, but tungsten retained useful strength even at 4,000 °F. But that metal could not be welded, while no coating could protect it against oxidation. Attention then turned to nonmetallic materials, including ceramics.
Ceramics of interest existed as oxides such as silica and magnesia, which meant that they could not undergo further oxidation. Magnesia proved to be unsuitable because it had low thermal emittance, while silica lacked strength. However, carbon in the form of graphite showed clear promise. It held considerable industrial experience; it was light in weight, while its strength actually increased with temperature. It oxidized readily but could be protected up to 3,000 °F by treating it with silicon, in vacuum and at high temperatures, to form a thin protective layer of silicon carbide. Near the stagnation point, the temperatures during reentry would exceed that level. This brought the concept of a nose cap with siliconized graphite as the primary material and with an insulated layer of a temperature-resistant ceramic covering its forward area. With graphite having good properties as a heat sink, it would rise in temperature uniformly and relatively slowly, while remaining below the 3,000 °F limit throughout the full time of the reentry.
Suitable grades of graphite proved to be available commercially from the firm of National Carbon. Candidate insulators included haf – nia, thoria, magnesia, ceria, yttria, beryllia, and zirconia. Thoria was the most refractory but was very dense and showed poor resistance to thermal shock. Hafnia brought problems of availability and of reproducibility of properties. Zirconia stood out. Zirconium, its parent metal, had found use in nuclear reactors; the ceramic was available from the Zirconium Corporation of America. It had a melting point above 4,500 °F, was chemically stable and compatible with siliconized graphite, offered high emittance with low thermal conductivity, provided adequate resistance to thermal shock and thermal stress, and lent itself to fabrication.[1044]
For developmental testing, Vought used two in-house facilities that simulated the flight environment, particularly during reentry. A ramjet, fueled with JP-4 and running with air from a wind tunnel, produced an exhaust with velocity up to 4,500 ft/sec and temperature up to 3,500 °F. It also generated acoustic levels above 170 decibels (dB), reproducing the roar of a Titan III booster and showing that samples under test could withstand the resulting stresses without cracking. A separate installation, built specifically for the Dyna-Soar program, used an array of propane burners to test full-size nose caps.
The final Vought design used a monolithic shell of siliconized graphite that was covered over its full surface by zirconia tiles held in place by thick zirconia pins. This arrangement relieved thermal stresses by permitting mechanical movement of the tiles. A heat shield stood behind the graphite, fabricated as a thick disk-shaped container made of coated TZM sheet metal and filled with Q-felt. The nose cap was attached to the vehicle with a forged ring and clamp that also were of coated TZM. The cap as a whole relied in radiative cooling. It was designed to be reusable; like the primary structure, it was to withstand four reentries under the most severe conditions permitted.[1045]
The backup Boeing effort drew on that company’s own test equipment. Study of samples used the Plasma Jet Subsonic Splash Facility, which created a jet with temperature as high as 8,000 °F that splashed over the face of a test specimen. Full-scale nose caps went into the Rocket Test Chamber, which burned gasoline to produce a nozzle exit velocity of 5,800 ft/sec and an acoustic level of 154 dB. Both installations were capable of long-duration testing, reproducing conditions during reentries that could last for 30 minutes.[1046]
The Boeing concept used a monolithic zirconia nose cap that was reinforced against cracking with two screens of platinum-rhodium wire. The surface of the cap was grooved to relieve thermal stress. Like its counterpart from Vought, this design also installed a heat shield that used Q-felt insulation. However, there was no heat sink behind the zirconia cap. This cap alone provided thermal protection at the nose, through radiative cooling. Lacking pinned tiles and an inner shell, its design was simpler than that of Vought.[1047]
Its fabrication bore comparison to the age-old work of potters, who shape wet clay on a rotating wheel and fire the resulting form in a kiln. Instead of using a potter’s wheel, Boeing technicians worked with a steel die with an interior in the shape of a bowl. A paper honeycomb, reinforced with Elmer’s Glue and laid in place, defined the pattern of stress-relieving grooves within the nose cap surface. The working material was not moist clay but a mix of zirconia powder with binders, internal lubricants, and wetting agents.
With the honeycomb in position against the inner face of the die, a specialist loaded the die by hand, filling the honeycomb with the damp mix and forming layers of mix that alternated with the wire screens. The finished layup, still in its die, went into a hydraulic press. A pressure of
27,0 psi compacted the form, reducing its porosity for greater strength and less susceptibility to cracks. The cap was dried at 200 °F, removed from its die, dried further, and then fired at 3,300 °F for 10 hours. The paper honeycomb burned out in the course of the firing. Following visual and x-ray inspection, the finished zirconia cap was ready for machining to shape in the attachment area, where the TZM ring-and-clamp arrangement waste anchor it to the fuselage.[1048]
The nose cap, outer panels, and primary structure all were built to limit their temperatures through passive methods: radiation and insulation. Active cooling also played a role, reducing temperatures within the pilot’s compartment and two equipment bays. These used a "water wall” that mounted absorbent material between sheet-metal panels to hold a mix of water and a gel. The gel retarded flow of this fluid, while the absorbent wicking kept it distributed uniformly to prevent hotspots.
During reentry, heat reached the water walls as it penetrated into the vehicle. Some of the moisture evaporated as steam, transferring heat to a set of redundant water-glycol loops that were cooled by liquid hydrogen from an onboard supply. A catalytic bed combined the stream of warmed hydrogen with oxygen that again came from an onboard supply. This produced gas that drove the turbine of Dyna-Soar’s auxiliary power unit, which provided both hydraulic and electric power to the craft.
A cooled hydraulic system also was necessary, to move the control surfaces as on a conventional airplane. The hydraulic fluid operating temperature was limited to 400 °F by using the fluid itself as an initial heat-transfer medium. It flowed through an intermediate water – glycol loop that removed its heat by being cooled with hydrogen. Major hydraulic components, including pumps, were mounted within an actively cooled compartment. Control-surface actuators, along with associated valves and plumbing, were insulated using inch-thick blankets of Q-felt. Through this combination of passive and active cooling methods, the Dyna-Soar program avoided a need to attempt to develop truly high-temperature arrangements, remaining instead within the state of the art.[1049]
Specific vehicle parts and components brought their own thermal problems. Bearings, both ball and antifriction, needed strength to carry mechanical loads at high temperatures. For ball bearings, the cobalt – base superalloy Stellite 19 was known to be acceptable up to 1,200 °F. Investigation showed that it could perform under high load for short durations at 1,350 °F. Dyna-Soar nevertheless needed ball bearings qualified for 1,600 °F and obtained them as spheres of Rene 41 plated with gold. The vehicle also needed antifriction bearings as hinges for control surfaces, and here there was far less existing art. The best available bearings used stainless steel and were suitable only to 600 °F, whereas Dyna – Soar again faced a requirement of 1,600 °F. A survey of 35 candidate materials led to selection of titanium carbide with nickel as a binder.[1050]
Antenna windows demanded transparency to radio waves at similarly high temperatures. A separate program of materials evaluation led to selection of alumina, with the best grade being available from the Coors Porcelain Company.[1051]
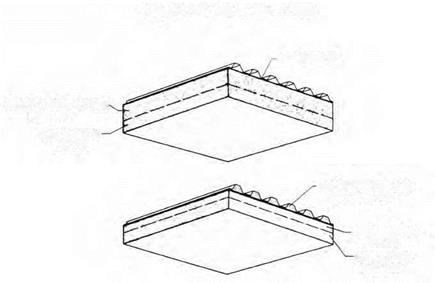 ABLATIVE HEAT SHIELDS
ABLATIVE HEAT SHIELDS
T —500 °F
WATER COOLING
(T<150 °F)
INSULATION
ABLATION
LAYER
NASA concepts for passive and actively cooled ablative heat shields, 1960. NASA.
The pilot needed his own windows. The three main ones, facing forward, were the largest yet planned for a piloted spacecraft. They had double panes of fused silica, with infrared-reflecting surfaces on all surfaces except the outermost. This inhibited the inward flow of heat by radiation, reducing the load on the active cooling of the pilot’s compartment. The window frames expanded when hot; to hold the panes in position, those frames were fitted with springs of Rene. The windows also needed thermal protection so they were covered with a shield of D-36. It was supposed to be jettisoned following reentry, around Mach 5, but this raised a question: what if it remained attached? The cockpit had two other windows, one on each side, which faced a less severe environment and were to be left unshielded throughout a flight. Over a quarter century earlier, Charles Lindbergh had flown the Spirit of St. Louis across the North Atlantic from New York to Paris using just side vision and a crude periscope. But that was a far cry from a plummeting lifting reentry vehicle. Now, test pilot Neil Armstrong flew Dyna-Soar – like approaches and landings in a modified Douglas F5D-1 fighter with side vision only and showed it was still possible.[1052]
The vehicle was to touch down at 220 knots. It lacked wheeled landing gear, for inflated rubber tires would have demanded their own cooled compartments. For the same reason, it was not possible to use a conventional oil-filled strut as a shock absorber. The craft therefore deployed tricycle landing skids. The two main skids, from Goodyear, were of Waspalloy nickel steel and mounted wire bristles of Rene 41. These gave a high coefficient of friction, enabling the vehicle to skid to a stop in a planned length of 5,000 feet while accommodating runway irregularities. In place of the usual oleo strut, a long rod of Inconel stretched at the moment of touchdown and took up the energy of impact, thereby serving as a shock absorber. The nose skid, from Bendix, was forged from Rene 41 and had an undercoat of tungsten carbide to resist wear. Fitted with its own energy-absorbing Inconel rod, the front skid had a reduced coefficient of friction, which helped to keep the craft pointing straight ahead during slide-out.[1053]
Through such means, the Dyna-Soar program took long strides toward establishing hot structures as a technology suitable for operational use during reentry from orbit. The X-15 had introduced heat sink fabricated from Inconel X, a nickel steel. Dyna-Soar went considerably further, developing radiation-cooled insulated structures fabricated from Rene 41 and from refractory materials. A chart from Boeing made the point that in 1958, prior to Dyna-Soar, the state of the art for advanced aircraft structures involved titanium and stainless steel, with temperature limits of 600 °F. The X-15 with its Inconel X could withstand temperatures above 1,200 °F. Against this background, Dyna-Soar brought substantial advances in the temperature limits of aircraft structures.[1054]










